Full HTML
Galectin-3 expression by thyroid neoplasms in a center in southwest Nigeria
Ademola Idowu Soremekun1, Olaejirinde Olaniyi Olaofe1, Akinwumi Oluwole Komolafe1
Author Affiliation
1Lecturer, Department of Morbid Anatomy and Forensic Medicine, Obafemi Awolowo University Teaching Hospital, Ile-Ife, Osun State, Nigeria
Abstract
Background: Galectin-3 (GAL-3), which is known to be expressed in certain normal tissues and is implicated in tumor progression and metastasis, serves as a valuable marker for distinguishing malignant thyroid lesions from benign thyroid lesions. This study aimed to assess the clinical and histopathological features of thyroid neoplasms at OAUTHC Ile Ife utilizing GAL-3 immunohistochemistry and to compare these findings with those of studies conducted in other regions of the world. Materials and Methods: Histology slides from 56 patients with thyroid neoplasms spanning a 20-year period were subjected to immunohistochemical staining with monoclonal antibodies against GAL-3. Evaluation was based on the 2022 World Health Organization Classification of Thyroid Neoplasms, categorizing marker expression as follows: 1+ (10–25% stained cells), 2+ (26–50% stained cells), 3+ (51–75% stained cells), and 4+ (>75% stained cells). Negative staining indicated no staining, while localized staining (focal) represented <50% of cells stained (1+ or 2+), and diffuse staining indicated >50% of cells stained (3+ or 4+). Results: A total of 56 patients with neoplastic thyroid disease were studied, including 15 males and 41 females. Among the 16 benign neoplastic lesions, four (25.0%) exhibited focal GAL-3 expression, while 12 (75.0%) showed no expression. Among the forty malignant neoplastic lesions, five (12.5%) tested negative for the GAL-3 immunomarker, while 35 (87.5%) demonstrated positive expression to varying degrees. Among the positive malignant lesions, two (5.0%) displayed focal GAL-3 expression, while 33 (82.5%) exhibited diffuse staining of tumor cells. The diffuse and strong expression of GAL-3 was significantly greater in malignant thyroid neoplasms than in benign neoplasms (p<0.05). The sensitivity and specificity of GAL-3 were 88% and 75%, respectively. Conclusion: Our findings align with the previous research indicating that diffuse and strong immunohistochemical expression of GAL-3 is indicative of thyroid gland malignancy. GAL-3 serves as a valuable tool in diagnosing thyroid neoplasms, particularly in cases where histomorphologic characteristics are inconclusive or unclear.
DOI: 10.32677/yjm.v3i1.4452
Keywords: Antibody, Galectin-3, Immunohistochemistry, Neoplasm, Thyroid
Pages: 30-35
View: 5
Download: 9
DOI URL: https://doi.org/10.32677/yjm.v3i1.4452
Publish Date: 11-05-2024
Full Text
INTRODUCTION
Galectin-3 (GAL-3) belongs to the animal lectin family, which binds to β-galactosides and is recognized for its involvement in the metastasis and progression of tumors [1]. In humans, it is encoded by the LGALS3 gene [2]. GAL-3 is distributed in various normal tissues, including folliculostellate cells of the adenohypophysis, peripheral nerves, and endometrial cells [3]. Neoplastic disorders, such as parathyroid, lung, liver, and colon cancers, as well as thyroid tumors, have been found to be positive for GAL-3 [3]. GAL-3 plays crucial roles in cell‒cell/cell-matrix adhesion, cell growth, neoplastic transformation/spread, cell cycle regulation/apoptosis, and cell repair processes [3]. Moreover, it is expressed by human macrophages, neutrophils, mast cells, and Langerhans cells [4]. GAL-3 typically shows strong and diffuse cytoplasmic staining in various carcinomas, particularly in well-differentiated follicular thyroid carcinomas (FTCs) [4]. It has emerged as a valuable marker for differentiating malignant thyroid lesions from benign lesions [1].Despite its classification as a rare cancer, thyroid cancer ranks as the 19th most common cancer in the UK, with 880 new cases diagnosed in men and 2400 in women in 2013 [5]. The majority of thyroid cancer cases in the UK occur in individuals aged 50 and above [5]. In Nigeria, researchers have reported numerous cases of thyroid cancer, often diagnosed without ancillary tests [6,7]. While histomorphological examination of routine hematoxylin and eosin (H&E)-stained sections is usually sufficient for diagnosing thyroid neoplasms, challenges arise in differentiating between benign and malignant lesions due to equivocal features [8,9]. Notably, interobserver or intraobserver disagreements in diagnosing follicular thyroid lesions are common, even among expert pathologists [8,9]. These diagnostic uncertainties directly impact patient management and prognostication [8,9]. Consequently, immunohistochemistry and other ancillary investigations play a crucial role in diagnosing thyroid neoplasms, especially in challenging cases.This study aimed to review the clinical and histopathological characteristics of thyroid neoplasms observed at OAUTHC Ile Ife utilizing GAL-3 as an immunohistochemical marker and to compare the findings with those of studies conducted in other regions.
MATERIALS AND METHODS
Study Design, Setting, and Population
A retrospective analysis was conducted on all consecutive cases of neoplastic thyroid lesions documented in the Department of Morbid Anatomy and Forensic Medicine’s daybook at OAUTHC, Ile-Ife, between July 1, 1996, and June 30, 2016. Histomorphologic assessment utilizing H&E-stained slides, either alone or combined with specific stains, was employed for diagnosing thyroid neoplasms during this period. Slides from patient archives were retrieved and examined according to the World Health Organization (WHO) 2022 Classification of Thyroid Neoplasms. In instances where slides could not be found in the archive, new slides were generated from paraffin-embedded blocks to review prior diagnoses. The specimens utilized in the study were sourced from patients in the southwest region of Nigeria.
Sample Size and Sampling Technique
All consecutive patients within the study period were included (complete enumeration) with adoption of a purposive sampling technique. Patients with insufficient or inappropriate tissue or incomplete patient biodata were excluded from the analysis.
Summary of GAL-3 Antibody Utilization
For each tissue block sample, regardless of the original H&E diagnosis, exposure to the GAL-3 antibody (BSB, clone 9C4) was conducted using heat-induced epitope retrieval. Tissue specimen sections were attached to charged slides and submerged in citrate buffer (Ph = 6.0) diluted to a 1:10 ratio with distilled water. Slides were subjected to a gradual temperature increase in a microwave until reaching 90°C, followed by cooling in cold water for 20 min before reinsertion into new citrate. Subsequent cleaning with phosphate buffer solution (PBS) was performed, and biochemical reactions for each batch were assessed for integrity using positive and negative controls. Skin tissue served as the positive control according to the manufacturer’s instructions. Following blocking of endogenous peroxidase and incubation with a suitably diluted mouse anti-GAL-3 antibody, labeled polymer horseradish peroxidase and conjugated anti-mouse secondary antibody were applied. The samples were incubated, rinsed with PBS, and diaminobenzidine solution was added, followed by subsequent incubation in a humidity chamber. Hematoxylin was utilized as a counterstain. Patients were examined independently without access to initial histologic diagnoses. The slides were evaluated according to the 2022 WHO Classification of Thyroid Neoplasms. Marker expression was categorized as follows: 1+ (10–25% stained cells), 2+ (26–50% stained cells), 3+ (51–75% stained cells), and 4+ (>75% stained cells). Negative staining indicated no staining, localized staining represented <50% of cells stained (1+ or 2+), and diffuse staining indicated >50% of cells stained (3+ or 4+) [10].
Statistical Analysis
Frequency tables, descriptive statistics, and comparative statistics were used to present the data after they were analyzed using SPSS 20. To compare nonquantitative and categorical variables, the Chi-square test was used. At p<0.05, the threshold for statistical significance was established. Next, calculations were made for sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Ethical Approval
The Obafemi Awolowo University Teaching Hospital’s Ethics and Research Committee granted approval for the study in Ile-Ife
RESULTS
In this study, a total of 56 patients with neoplastic thyroid disease — 15 males and 41 females — were eligible for inclusion. Twenty-four patients were excluded due to insufficient or inadequately stored tissues. The sole benign thyroid tumor identified in the study was follicular adenoma (FA). Among the malignant thyroid cases, FTC, medullary thyroid carcinoma (MTC), papillary thyroid carcinoma (PTC)-follicular variant (FV), and PTC were observed. Of the cases analyzed, 16 (28.6%) were benign, while 40 (71.4%) were malignant, resulting in a benign-to-malignant ratio of 1:2.5. The male-to-female ratio among the patients studied was 1:2.7. Thirteen of the benign thyroid tumors (FA) were identified in females, while three were identified in males. In women, malignant thyroid tumors were found to be 2.2 times more common than benign tumors. The prevalence and sex distribution of the various histological categories of thyroid neoplasms are illustrated in Fig. 1.
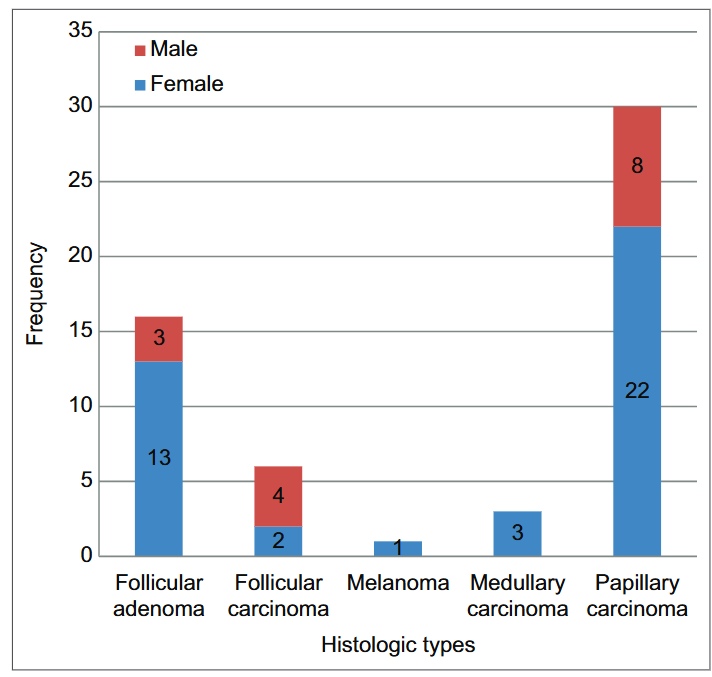
Figure 1: Frequency distribution of the histological types in both sexes
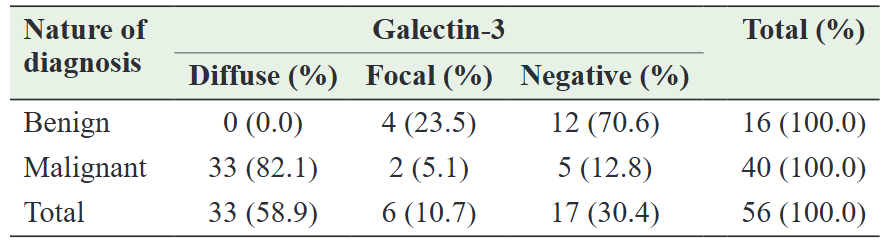
The study encompassed a varied age range, spanning from 16 to 83 years. Among the 56 cases of thyroid neoplasms, approximately 70% (40) occurred in individuals aged between 20 and 49 years. The mean age across all patients was 39.75 years. Malignant thyroid neoplasms predominantly manifested between the ages of 30 and 49, with a mean age of 42.3 years (standard deviation [SD]=15.5 years), while benign thyroid neoplasms were more prevalent among individuals aged 20–29 years, with a mean age of 33.7 years (SD=12.4 years).Among the patients studied, 17 (30.4%) exhibited negative GAL-3 expression, while 39 (69.6%) displayed varying degrees of positivity. GAL-3-positive cases primarily demonstrated cytoplasmic staining, with some instances also featuring nuclear staining. Within the subset of positive cases, six (10.7%) exhibited focal staining of tumor cells, while 33 (58.9%) displayed diffuse staining. A statistically significant relationship was observed between GAL-3 expression and patient class (p<0.05). Further details regarding GAL-3 staining among the neoplastic thyroid patients are provided in Table 1.
Table 1: Galectin-3 staining pattern in benign and malignant tumors
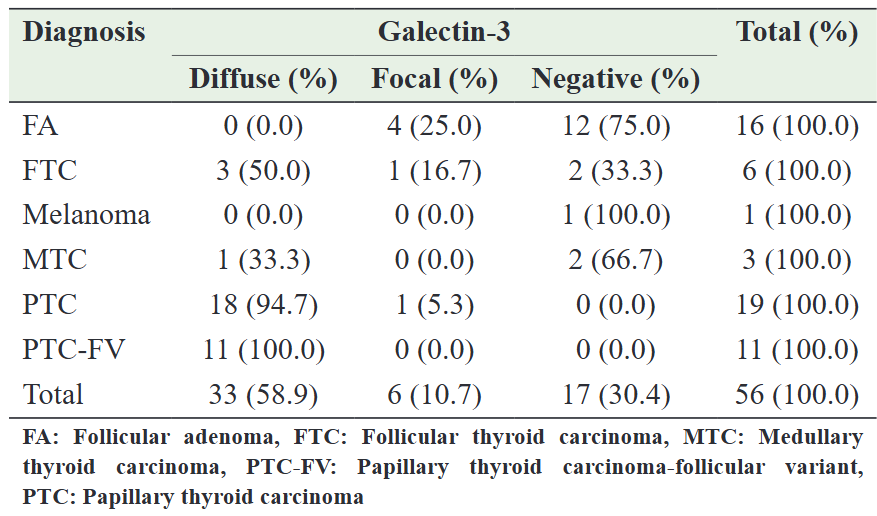
Among the benign neoplastic lesions, four (25.0%) exhibited focal GAL-3 expression, while 12 (75.0%) showed no expression. Regarding malignant neoplastic lesions, five (12.5%) tested negative for the GAL-3 immunomarker, whereas 35 (87.5%) demonstrated positive expression to varying degrees. Among the positive malignant lesions, two (5.0%) displayed focal GAL-3 expression, while 33 (82.5%) exhibited diffuse staining of tumor cells. Overall, seven (17.5%) thyroid cancers displayed either no expression or focal expression of GAL-3. The detailed data on GAL-3 expression in both benign and malignant patients are provided in Table 2.
Table 2: Expression of galectin-3 in neoplastic thyroid specimens
The sensitivity, specificity, PPV, and NPV of GAL-3 in testing for malignancy in thyroid tumors were 88%, 75%, 90%, and 71%, respectively. However, if only diffusely stained tumors are considered to be positive, the sensitivity, specificity, PPV, and NPV of GAL-3 will be 82.5%, 100%, 100%, and 70%. Positive staining for GAL-3 was observed in all thirty patients with PTC. Among these patients, one (a classic PTC) exhibited focal expression of the antibody, while the remaining twenty-nine PTCs (comprising 18 classic PTCs and 11 FVs of PTCs) displayed diffuse expression of the antibody. H&E-stained sections depicting a patient with PTC, along with the diffuse expression of GAL-3, are shown in Figs. 2 and 3, respectively. Fig. 4 shows negative GAL-3 staining in the papillary structures of a FA.Two of the FTC patients were negative for GAL-3, while four tested positive. Among the positive FTC tumors, one displayed focal positivity, and three exhibited diffuse staining. Furthermore, the study included four cases of rare cancers, one case of melanoma (probably metastatic) and three cases of medullary thyroid carcinoma (MTC). We could not determine the origin of the melanoma. GAL-3 expression was not detected in two of the three MTC patients, whereas the third patient exhibited widespread GAL-3 expression. The solitary melanoma patient did not express GAL-3.
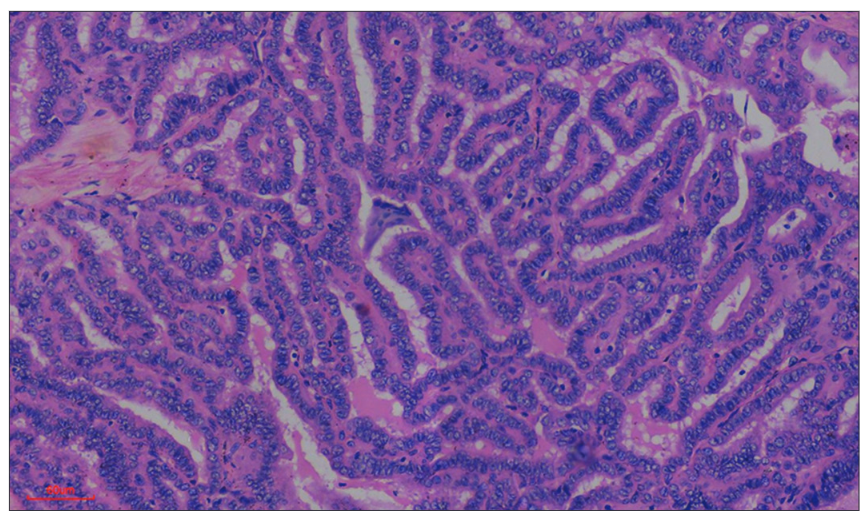 | 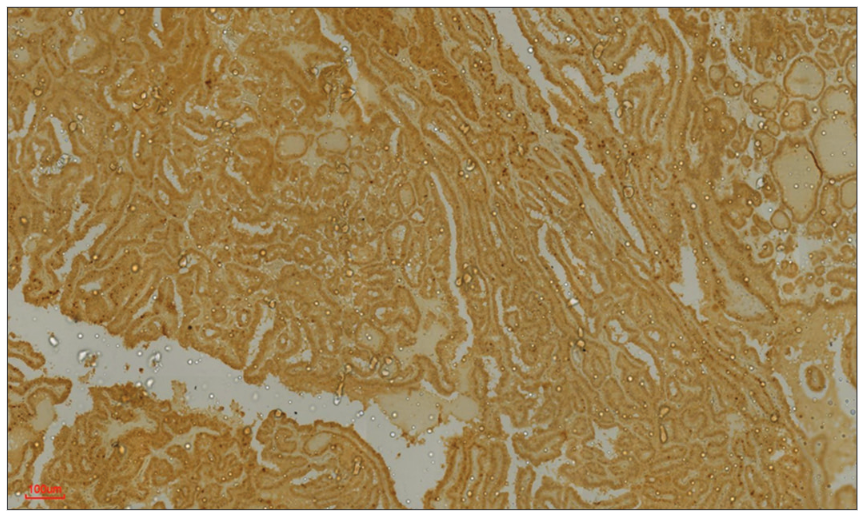 | 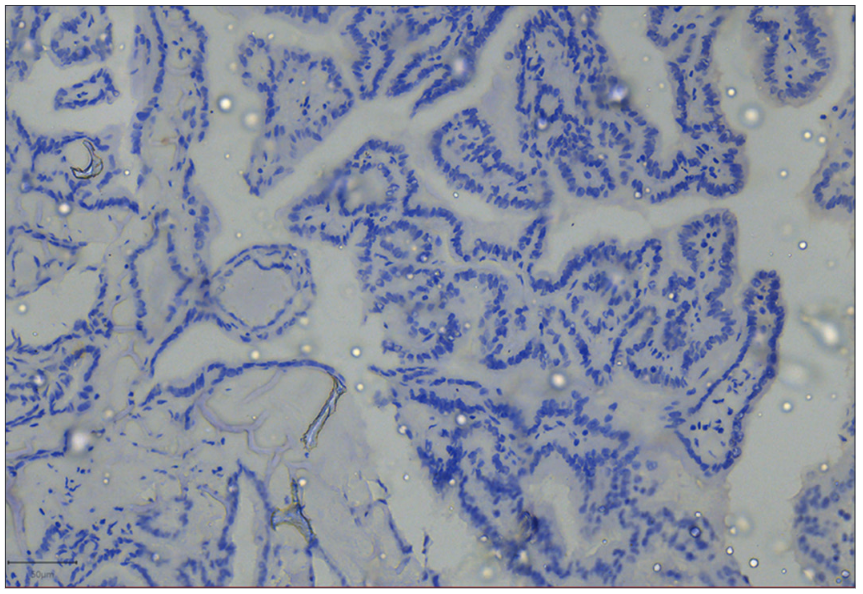 | |
| Figure 2: Papillary carcinoma of the thyroid. Photomicrographshowing the proliferation of complex papillary structures in a case of classic papillary carcinoma of the thyroid in a 30-year-old female | Figure 3: Galectin-3 (GAL-3) positivity in papillary thyroid carcinoma. Photomicrograph of the same patient above showing diffuse staining (almost 100%, 4+) for GAL-3 | Figure 4: Galectin-3 (GAL-3) negativity in papillary structures of a follicular adenoma. Photomicrograph showing no staining for GAL-3 in papillary structures of a follicular adenoma. The cells are devoid of nuclear features of papillary thyroid carcinoma. GAL-3 was useful for confirming benignity in this patient |
DISCUSSION
In our study, malignant lesions were nearly 3 times more prevalent than benign lesions, suggesting a greater frequency of diagnosing malignant tumors within our department. This finding is consistent with the study conducted by Adeniyi et al., which also reported a greater incidence of malignant cases than benign cases [11]. While many organs typically exhibit a greater frequency of benign tumors than malignant ones, the observed disparity in our thyroid specimens may be attributed to benign tumors being embedded within nodular colloid goiters. In addition, it is possible that patients with benign masses may not seek medical attention unless symptomatic, given that apart from commonly noticed neck masses, the lesions are often asymptomatic. Further studies are warranted to specifically elucidate the reasons underlying the disparity in these ratios before definitive conclusions can be drawn. In our study, there was a predominance of females, with approximately 3 times as many females diagnosed with thyroid tumors as males. This finding aligns with numerous studies on thyroid conditions and neoplastic tumors [6,12-14], indicating a greater incidence of thyroid neoplasms in women. Several studies have implicated various sex hormonal factors in the development of thyroid neoplasms, including increased parity, later age at first pregnancy, fertility issues, and oral contraceptive use [15,16].
In addition, the presence of estrogen receptors in some thyroid tumors may partly account for the relatively greater frequency of thyroid tumors in females.Our study also revealed that the peak age for malignant thyroid neoplasms occurred approximately a decade after the peak age for benign thyroid neoplasms. This observation is consistent with findings reported by Ariyibi et al. in Ibadan [6]. It is well established that malignant tumors in many organs tend to occur at older ages compared to benign tumors. According to the theory of carcinogenesis, the potential for more aggressive behaviors arises after the accumulation of mutations over time. Thus, malignant tumors are typically observed in older individuals who may have accumulated more mutations following prolonged exposure to carcinogens.An earlier study conducted by Lawal et al. at Obafemi Awolowo University Teaching Hospital, Ile-Ife, which focused on surgical cases of malignant thyroid swelling between 1983 and 1993, reported follicular carcinoma as the most prevalent type of thyroid cancer [17]. However, our current study revealed a shift, with papillary carcinoma emerging as the most common thyroid malignancy. It is worth noting that the catchment areas of Obafemi Awolowo University Teaching Hospital still primarily comprise southwestern towns and states surrounding Ile-Ife, characterized by highlands consisting of pre-Cambrian granite and low iodine content [17]. Our conjecture is that the introduction of iodine supplements in salt, sugar, and other consumables over 30 years ago may have influenced the pattern of thyroid cancers, resembling that of iodine-sufficient regions, which often exhibit a higher prevalence of papillary thyroid cancer.In this study, FAs did not exhibit diffuse GAL-3 staining. This observation aligns with the findings of Nechifor-Boila in Romania, where none of the FAs tested positive for GAL-3 [10]. However, contrasting results were documented by Prasad et al. and Scognamiglio et al., who reported positive staining in 9.5% and 18% of cases, respectively [18,19].The calculated sensitivity and specificity of GAL-3 for differentiating benign from malignant neoplasms were 88% and 75%, respectively. The sensitivity was reduced to 82.5%, while the specificity increased to 100% if only diffusely stained tumors were considered positive. This sensitivity is comparable to the findings of Liu and Lin, who reported a sensitivity of 84.6%. However, it falls short of the values obtained by Fischer and Asa and Park et al. [1,4,20]. Our findings, in conjunction with those of other researchers, collectively suggest the relatively high sensitivity and specificity of GAL-3 in identifying the malignant nature of thyroid tumors. We found that strong diffuse staining of tumor cells by GAL-3 is highly specific (specificity, 100%) in the identification of malignant thyroid tumors. In our study, nearly all patients with papillary carcinoma exhibited diffuse staining of GAL-3 by tumor cells, with only one tumor showing focal expression. This finding is consistent with Scognamiglio’s et al. observation in New York, United States, where 96% of cases displayed positive staining of tumor cells for GAL-3. Park et al. reported a slightly lower value of 94.0% [19,20]. These findings suggest that pathologists should be weary of diagnosing PTC in patients with seemingly papillary thyroid lesions who are negative for GAL-3. These findings also indicate that GAL-3 may be valuable for identifying the primary site of metastatic papillary tumors in bones or lymph nodes GAL-3-negative metastatic papillary tumors are unlikely to be papillary carcinomas of the thyroid.Furthermore, approximately half of the follicular carcinomas demonstrated diffuse GAL-3 staining, 33.3% were negative, and 16.7% exhibited focal positivity. Prasad et al. and Saleh et al. reported positivity values of 67.0% and 81.8%, respectively [18,21]. All these findings suggest that GAL-3 is of limited value in distinguishing benign and malignant follicular tumors of the thyroid. In our study, we encountered three cases of medullary carcinoma and one case of melanoma (probably metastatic), all of which are rare. Most of the patients with medullary carcinoma in our study were negative for GAL-3 expression. This observation aligns with the findings of Xu et al., who reported a 37.5% expression of GAL-3 in MTCs. However, Bartolazzi et al. reported a higher percentage (43.0%) of GAL-3 expression in MTCs [22,23]. Our findings, along with those of other researchers, suggest that GAL-3 cannot reliably identify MTCs. Nonetheless, it may offer partial utility in distinguishing MTC from other malignant thyroid lesions, particularly when combined with other immunomarkers. It is important to note, however, that due to the rarity of MTC, which was included in our study, the number of patients available for analysis was limited. Given the retrospective nature of our study, limitations are inherent, particularly regarding the availability of properly stored formalin-fixed paraffin-embedded tissues. Long-term storage may have resulted in some loss of antigenicity. In addition, the relatively small number of cases for certain tumor types restricts the extent of inference that can be drawn from our study outcomes. A multicenter study with the aggregation of a larger number of patients may yield more statistically significant findings.
CONCLUSION
Our findings are similar to reports from various researchers. Our study highlights the gender disparity in thyroid neoplasms, with a greater prevalence among women and a greater likelihood of malignancy in men. GAL-3 has emerged as a significant biomarker associated with the benign or malignant nature of thyroid neoplasms. Strong and diffuse immunohistochemical expression of GAL-3, which is observed in more than 50% of tumor cells (+3 or +4), serves as a robust indicator of thyroid gland malignancy, particularly in papillary tumors. This biomarker holds promise as a valuable tool for identifying the primary sites of metastatic papillary adenocarcinomas, particularly in cases where histomorphologic characteristics are inconclusive. However, the interpretation of immunohistochemical staining in thyroid neoplasms warrants caution, as occasional focal positive staining may occur in benign neoplastic cases. Therefore, robust and diffuse staining of tumor cells remains the optimal predictor of malignancy. GAL-3 expression plays a pivotal role in characterizing thyroid neoplasms and has potential for enhancing diagnostic accuracy and clinical management.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the reported work, participating in various aspects such as conception, study design, implementation, data collection, analysis, and interpretation, as well as contributing to drafting, revising, and critically reviewing the article, and ultimately approving the final version for publication
References
1. Liu H, Lin F. Application of immunohistochemistry in thyroid pathology. Arch Pathol Lab Med. 2015;139(1):67–82.
2. Raz A, Carmi P, Raz T, et al. Molecular cloning and chromosomal mapping of a human galactoside-binding protein. Cancer Res. 1991;51(8):2173–2178.
3. Pernick N. Galectin 3 [Internet]. Pathology outlines. 2013 [cited 2017 May 1]. Available from: http//www.pathologyoutlines.com/stains and molecular markers/galectin 3.
4. Fischer S, Asa SL. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med. 2008;132(3):359–372.
5. CancerResearchUK. Thyroid cancer statistics [Internet]. Cancer Research UK. 2013 [cited 2016 Sep 22]. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/thyroid-cancer.
6. Ariyibi O, Duduyemi B. Histopathological Patterns of Thyroid Neoplasms in Ibadan Nigeria: A Twenty Year Retrospective Study. Int J Trop Dis Heal. 2013;3(2):148–156.
7. Olatoke S, Ajape A, Rahman G, et al. Thyroid Malignancies in Ilorin, Nigeria: A Clinico-pathological Review. J Surg Surg Sci. 2010;1(4):108–112.
8. Hirokawa M, Carney JA, Goellner JR, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002;26(11):1508–1514.
9. Franc B, De La Salmonière P, Lange F, et al. Interobserver and Intraobserver Reproducibility in the Histopathology of Follicular Thyroid Carcinoma. Hum Pathol. 2003;34(11):1092–1100.
10. Nechifor-Boilǎ A, Cǎtanǎ R, Loghin A, et al. Diagnostic value of HBME-1, CD56, Galectin-3 and Cytokeratin-19 in papillary thyroid carcinomas and thyroid tumors of uncertain malignant potential. Rom J Morphol Embryol. 2014;55(1):49–56.
11. Adeniyi KA, Anjorin AS, Ogunsulire IA. Histological patterns of thyroid diseases in a Nigerian Population. Nig. Qt. J. Hosp. Med. J. 1998;8(4):241-244.
12. Tsegaye B, Ergete W. Histopathologic pattern of thyroid disease. East Afr Med J. 2003;80:525–528.
13. Der E, Quayson S, Clegg-Lamptey J, et al. Thyroid Disorders in Accra, Ghana: A Retrospective Histopathological Study at the Korle-Bu Teaching Hospital. J Med Biomed Sci. 2013;2(1):1–7.
14. Ijomone E, Duduyemi B, Udoye E, et al. Histopathological review of thyroid diseases in southern Nigeria- a ten year retrospective study.12. J Med Med Sci 2014;5(6)127-132. 2014;5(6):127–32.
15. La Vecchia C, Ron E, Franceschi S, et al. A pooled analysis of case‒control studies of thyroid cancer. III. Oral contraceptives, menopausal replacement therapy and other female hormones. Cancer Causes Control. 1999;10(2):157–166.
16. Memon A, Darif M, Al-Saleh K, et al. Epidemiology of reproductive and hormonal factors in thyroid cancer: Evidence from a case‒control study in the Middle East. Int J Cancer. 2002;97(1):82–89.
17. Lawal O, Agbakwuru A, Olayinka OS, et al. Thyroid malignancy in endemic nodular goitres: prevelence, pattern and treatment. Eur J Surg Oncol. 2001;27:157-161.
18. Prasad ML, Pellegata NS, Huang Y, et al. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005;18(1):48–57.
19. Scognamiglio T, Hyjek E, Kao J et al. Diagnostic usefulness of HBME1, galectin-3, CK19, and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am J Clin Pathol. 2006;126(5):700–708
20. Park YJ, Kwak SH, Kim DC, et al. Diagnostic Value of Galectin-3, HBME-1, cytokeratin 19, high molecular weight cytokeratin, cyclin d1 and p27kip1 in the differential diagnosis of thyroid nodules. J Korean Med Sci. 2007;22:621-628.
21. Saleh HA, Jin B, Barnwell J, et al. Utility of immunohistochemical markers in differentiating benign from malignant follicular-derived thyroid nodules. Diagn Pathol. 2010;5:9.
22. Xu XC, El-Naggar AK, Lotan R. Differential Expression of Galectin-1 and Galectin-3 in Thyroid Tumours. Potential Diagnostic Implications. Am J Pathol. 1995;147(3):815-822.
23. Bartolazzi A, Gasbarri A, Papotti M, et al. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet. 2001;357:1644–1650.
