Full HTML
Updated review on malignancy-associated venous thromboembolism: Pathogenesis and comparison between various therapeutic modalities
Elmukhtar Habas1, Ala Habas2, Amnna Rayani3, Kalifa Farfar4, Eshark Habas5, Jamal Alfitori1, Mehdi Arrayes1, Aml Habas6, Abdel-Monem Badawi Yousif7, Abdel-Naser Elzouk1
Author Affiliation
1Senior Consultant, Department of Medicine
2Medical Student
3Senior Consultant, Department of Pediatrics, Tripoli Central Hospital
4Senior Consultant, Department of Medicine, Al Wakra Hospital, Al Wakrah, Qatar
5Medical Resident, Department of Medicine
6Medical Researcher, Department of Pediatrics, Pediatric Hospital, Tripoli, Libya
7Pharmacist, Department of Pharmacy, Hamad General Hospital, Doha, Qatar
Abstract
Venous thromboembolism (VTE) is one of the life-threatening complications in cancer patients, the incidence of which is affected by the patient and malignancy-related variables. Location, type, therapeutic route, stage, grade, and non-supportive treatment of the cancer are the most important VTE risk factors. Patient age, ethnicity, and concomitant genetic or acquired comorbidities or thrombophilias are known risk factors for VTE in cancer. All high-risk cancer patients admitted to hospitals or treated as outpatients should receive VTE prophylaxis. Low molecular weight heparin (LMWH) is the main treatment for active malignant VTE. Vitamin K antagonists and non-vitamin K-dependent oral anticoagulants are used in stable, nonbleeding cancer patients. Anticoagulation should be continued until the cancer is treated or at least controlled. Over the past two decades, randomized clinical and observational trials have improved the pathogenesis and therapeutic knowledge of VTE, but many challenges remain. The lack of an optimal primary prophylaxis method for inpatients and outpatients in oncology and the care of cancer-associated VTE in standard and high-bleeding risk groups are examples for which more clinical research on cancer-associated thrombosis is necessary to address these issues. In this review, we describe the pathogenesis, factors that increase the risk of VTE, and the comparison between the effectiveness of available anticoagulants in the treatment and prevention of VTE in cancer patients.
DOI: 10.32677/yjm.v3i1.4547
Keywords: Anticoagulation in malignancy, Cancer and direct oral anticoagulant, Low molecular weight heparin in malignancy, Venous thromboembolism in cancer, Vitamin K antagonist in cancer, Warfarin in malignancy
Pages: 4-20
View: 8
Download: 13
DOI URL: https://doi.org/10.32677/yjm.v3i1.4547
Publish Date: 11-05-2024
Full Text
Introduction
An imbalance in the natural process of blood clotting can significantly increase the risk of developing either a thrombosis or excessive bleeding [1]. In certain medical conditions such as disseminated intravascular coagulopathy or in patients with underlying cancer who develop a coagulopathy, there is a simultaneous increased risk of both thrombosis and bleeding. It is crucial to be vigilant and take necessary measures to prevent such complications in patients with a higher risk of thrombosis and bleeding.
Arterial and venous thromboembolism (VTE) can occur in cancer patients. The term "VTE" includes both deep vein thrombosis (DVT) and pulmonary embolism (PE), which are common in cancer, and its management justifies the focus of this review. Arterial and venous thrombi can restrict normal blood flow and lead to deleterious clinical consequences. The ability of blood to flow freely through arteries and veins is determined by complex homeostasis achieved by interactions between platelets, plasma proteins, inflammatory factors, cytokines, coagulation factors, and the integrity of the endothelial layer of veins and arteries [1]. Pregnancy, hormone therapy, contraceptives, and obesity are known to increase the risk of VTEs [2,3]. Moreover, the cumulative incidence of VTE after cancer diagnosis was found to be 11.1-fold higher than in patients without cancer [4].
In epidemiological research, VTE is often classified as either provoked or unprovoked. Provoked VTEs are the result of certain causes that occur during the three months before, such as immobility, trauma, surgery, malignancy, or hospitalization [5]. On the other hand, unprovoked occurrences happen without evidence of these causes being present [6,7]. The categorization of provoked or unprovoked VTEs, although beneficial for both epidemiological and therapeutic purposes is a subject of controversy [6,7]. Due to the complex nature of VTE, it might be difficult (or impossible) to pinpoint a specific trait that caused VTE or to classify a series of lesser thrombotic incidents that culminated in a VTE event as unprovoked. The 2019 guidelines from the European Society of Cardiology/European Respiratory Society for diagnosing and treating acute PE did not use the terms 'provoked' and 'unprovoked'. Instead, they emphasized that the long-term VTE recurrence risk is based on the underlying factors, with a risk of less than 3% per year considered low, 3-8% per year considered intermediate, and over 8% per year considered high [8].
In this review, we aim to discuss in detail the pathogenesis and factors that increase VTE, comparison between the effectiveness of available anticoagulants in the treatment and prevention of VTE, and the anticancer effects of anticoagulants currently used. To achieve the purpose of the review, various phrases and keywords (anticoagulation in malignancies, VTEs prevention in cancer, cancer-associated thrombosis, VTEs in malignancy, DOAC, VKA, and venous thromboembolism) were used to search PubMed, EMBASE, Scopus, Google Scholar, and Google search engine, looking for a recently published reviews and original articles on VTE in malignancy for the period between 1982 and 2024.
Venous Thromboembolism Epidemiology and Effects on Cancer Patients' Outcome
VTE is estimated to affect 1-2 persons/1,000 person-years in Europe and the US, with lesser rates in other world regions, despite the absence of VTE surveillance systems [5]. VTE is a frequent illness affecting > 1 in 12 subjects during their lifetime in the Western World [5]. An estimated 4-20% of cancer patients are likely to have VTE at some point, with the greatest occurrence rate seen shortly after diagnosis. Every year, 0.5% of individuals diagnosed with cancer will develop thrombosis, whereas the incidence rate in the general population is 0.1% [9]. VTE absolute incidence across all cancer types was 13.9/1000 person-years in Britain [10,11]. In hepatic and pancreatic malignancies, an East Asian study found a cancer-associated VTE incidence of 9.9/1000 person-years [12]. Moreover, about a fifth of subjects who develop a VTE attack die within 1 year, and the VTE in this group is usually due to a provoking condition.
VTE complications are more frequent among survivors [13]. Cancer patients make up around 20% of all VTE cases [14,15]. Cancer patients have a 5 to 7-fold higher likelihood of getting VTE [16]. In addition, individuals who acquire VTE at the time of cancer diagnosis or within the first year are likely to have a notably worse prognosis compared to cancer patients who do not experience VTE [17]. The occurrence of VTE in cancer patients is a significant and detrimental complication that harms their quality of life and decreases their chances of survival [18].
According to research, individuals with cancer had a 3 times greater chance of experiencing recurrent VTE and a 2 times greater chance of experiencing bleeding linked with anticoagulant treatment [19]. It was reported that gastrointestinal (GI) cancer patients have a higher VTE rate than non-GI cancer patients [20]. A study described that 220 GI cancer patients (27.3%) experienced either single or recurrent VTE attacks, with DVT accounting for 38.6% and PE for 20.5% [20].
VTE is linked to a significant burden of morbidities caused by enduring sequelae, including blood coagulation recurrence, post-thrombotic syndrome, and post-pulmonary embolism syndrome. Although there is awareness of several risk factors and triggers, about 33% of VTE incidents occur without any apparent inciting factor (unprovoked) [21]. Death occurs in approximately 6% of DVT cases and 12% of PE cases [22]. Unfortunately, VTE is one of the primary causes of mortality among cancer patients [23]. Cancer patients who also have VTE face a risk of death that is 10 times higher compared to individuals who just have VTE, and four times higher compared to cancer patients who do not have VTE [24]. VTE is the second cause of mortality in individuals with cancer [23,25]. Hence, it is crucial to prioritize VTE prevention and treatment for effective management of patients with cancer.
Factors Affecting VTE Pathogenesis in Malignancy
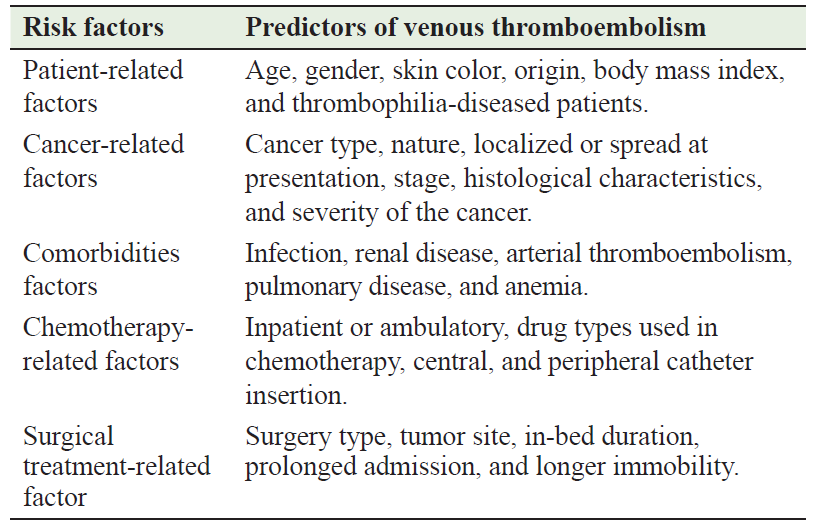
Table 1 summarizes the factors influencing the pathogenesis of VTE, particularly in malignancies. The incidence of VTE in malignancies is influenced by factors such as the primary location of the tumor, its stage, and histological features. The highest-risk cancers include pancreatic, lung, stomach, and brain cancers, as well as hematologic malignancies, while the lowest-risk cancers include prostate and breast cancers [26–29]. The likelihood of developing VTE increases proportionally to the severity of the cancer. The adjusted relative risk of VTE hospitalization in Danish population-based research was 2.9, 2.9, 7.5, and 17.1 for stage 1, 2, 3, and 4 cancers respectively [30]. Higher-grade tumors (grades 3 and 4) have twice the incidence of VTE compared to lower-grade tumors (grades 1 and 2) [31]. The incidence of VTE varies over the course of the disease in cancer patients. Within the first 6 months after diagnosis of colorectal cancer, the probability of developing VTE is 5 cases/100 person-years. This risk decreases to 1.4 cases/100 person-years between 7 and 12 months after diagnosis and decreases further to 0.6/100 person-years between 13 and 24 months after initial presentation [32]. Cancer treatment also affects a patient's susceptibility to VTE. For example, the susceptibility to VTE in oncologic surgery is 1.7 times higher than in non-cancer surgery [33]. In a population-based observational analysis of breast cancer, it was found that undergoing surgery was linked to a 2.2-fold higher VTE risk in the first month after being discharged. However, this risk decreased in the following months. The Rochester Epidemiology Project found that chemotherapy was linked to a 1.8-fold uprise in VTE incidence [34].
Chemotherapy in breast cancer patients was found to be linked to a 10.8-fold increase of VTE during treatment, although this risk returned to normal levels three months after treatment was finished. The use of hormonal therapy was found to be linked to 2.4 times higher VTE risk within the initial 3 months o f treatment with the degree of risk varying depending on the specific medication used. Tamoxifen exhibited a 5.5-fold higher risk, while aromatase inhibitors did not show any association with VTE. Treatment plans that include thalidomide or lenalidomide along with high-dose dexamethasone, with or without multi-agent chemotherapy, were associated with a higher feasibility of developing VTE. On the other hand, treatment plans that involve low-dose dexamethasone or bortezomib are associated with less VTE risk [35]. Although early research indicated that the bevacizumab (the vascular endothelial growth factor receptor - VEGFR) antagonist, that causes vasoconstriction of tumor supplying blood vessel) use was linked to a highe likelihood of VTE, a recent meta-analysis that considered the duration of treatment found no evidence of an increased risk [36]. There has been no observed rise in VTE when using other inhibitors of VEGFR [37]. Nevertheless, monoclonal antibodies that target the epidermal growth factor (EGF) receptor (EGFR) have been linked to two times greater VTE risk, whereas, EGFR tyrosine kinase inhibitors have not shown this association [38]. The EGF binds to the EGFR, dimerizing, activating, autophosphorylating it, and activating signaling pathways that promote proliferation [39]. The utilization of erythropoiesis stimulatory agents generally heightens the tendency of VTE [40]. Surgically treated cancer patients have a higher risk of developing VTEs due to varied reasons, including immobility, vessel injuries, surgical approach, and prolonged bedbound periods. Furthermore, in cancer, VTE risk was notably elevated when surgery was conducted within the two months following chemotherapy treatment [28].
Individual patient features also have an impact on VTE risk. Hospitalized cancer patients who are aged ≥ 65 years, especially females have shown a slightly higher tendency of developing VTE. Black patients had a 20% higher risk, while Asian patients had a reduced risk [40]. Ashrani and colleagues observed that obesity, defined as a body mass index of 35 kg/m2 was linked to a fourfold rise in risk [41]. The large case‐control study on VT (MEGA case-control study) found that individuals with factor V Leiden heterozygosity had a 100% increased VTE incidence [14]. Comorbid disorders such as infection, renal disease, arterial thromboembolism, pulmonary disease, and anemia increase the VTE rate in cancer patients [42].
Table 1: Patient, cancer, comorbidities, and therapy types used are risk factors for venous thromboembolism
Pathophysiology of Venous Thromboembolism
Figure 1 describes the pathophysiology of thromboembolism in different clinical settings. Thrombosis is characterized by the obstruction of the circulatory system's normal blood flow due to the formation of a thrombus within a blood vessel. A thrombus is composed of a plug-forming aggregate of platelets and red blood cells and a fibrin protein mesh that has been cross-linked. The underlying mechanisms of thrombosis are significantly influenced by Virchow's triad, which consists of endothelial injury or malfunction, hypercoagulability, and blood stasis inside the veins or arteries. The vascular wall's damage or malfunction results in the generation of pro-inflammatory, cytokines, prothrombotic, the growth of adhesion molecules, an elevation in tissue factor availability, and intensified platelet activation. Cytokines trigger an inflammatory interaction between leukocytes and endothelial cells. Thrombophilia patients (deficits in protein C & S, anticardiolipin antibody, and antithrombin III) are more susceptible for developing thrombosis. Recent surgery, inflammation, pregnancy, infection, estrogen-containing medications, morbid obesity, or smoking increase further thrombosis risk.
Inflammation is a natural response of the body to undesirable stimuli, such as external pathogens, infection, or damage to the endothelium cells. This response may occur in acute conditions, like as trauma or surgery, and chronic conditions, such as underlying inflammatory illnesses or peripheral vascular disease. Due to these stimuli, endothelial cells and leukocytes become activated, leading to adhesion molecule formation that ultimately triggers the development of blood clots [43]. The body's innate anticoagulants (protein C & S and antithrombin-III) inhibit the development of blood clots via an intricate regulatory process that ensures stability intravascularily. Furthermore, physiologically, the body has continued thrombolysis processes that lysis the small thromboses. Imbalances in the process of clot formation and dissolution, cause extensive intravascular thrombosis.
VTE often originates in the sinus of the veins' valves, where distinct characteristics of the valves increase the blood clot formation likelihood. These factors include aberrant and diminished circulation of blood, decreased shear stress, and hypoxia resulting in an endothelium that is intact but not functioning properly [44]. Furthermore, platelets and leukocytes tend to get ensnared in valve pockets [45]. All these mechanisms that engage in prevention and thrombus formation are altered by tumors in cancer patients. For example, tumor overgrowth, increased cellular part of the blood, and abnormal increase of blood protein content all may exert pressure on veins, delaying blood flow, and injuring the vascular endothelium, leading to venous stasis, which in turn promotes the formation of thrombosis.
Myocardial infarction and coronary heart disease prevalence is high in cancer [46]. The uppermost prevalence was reported during the first 6 to 12 months after cancer diagnosis [47–49], and the risk remains high even after 10 years [49]. Arterial thrombosis, along with the creation of microthrombi, usually begins when lipid plaques build up in the arterial walls, triggering the activation of platelets and inflammatory cells, as in coronary heart disease [50]. Platelets have a greater impact on arterial thrombosis formation compared to venous thrombosis formation. Therefore, antiplatelet medications are essential in preventing and treating arterial thrombosis. The early lipid plaques progress into fibrous plaques (atherosclerosis). As a consequenc, the fibrous plaque has the potential to rupture and detach away some plaques of the arterial endothelium, resulting in the discharge of extra pro-coagulating substances [50]. Atherosclerosis facilitates the stimulation of platelets, resulting in their adherence and aggregation, ultimately leading to clot formation [43].
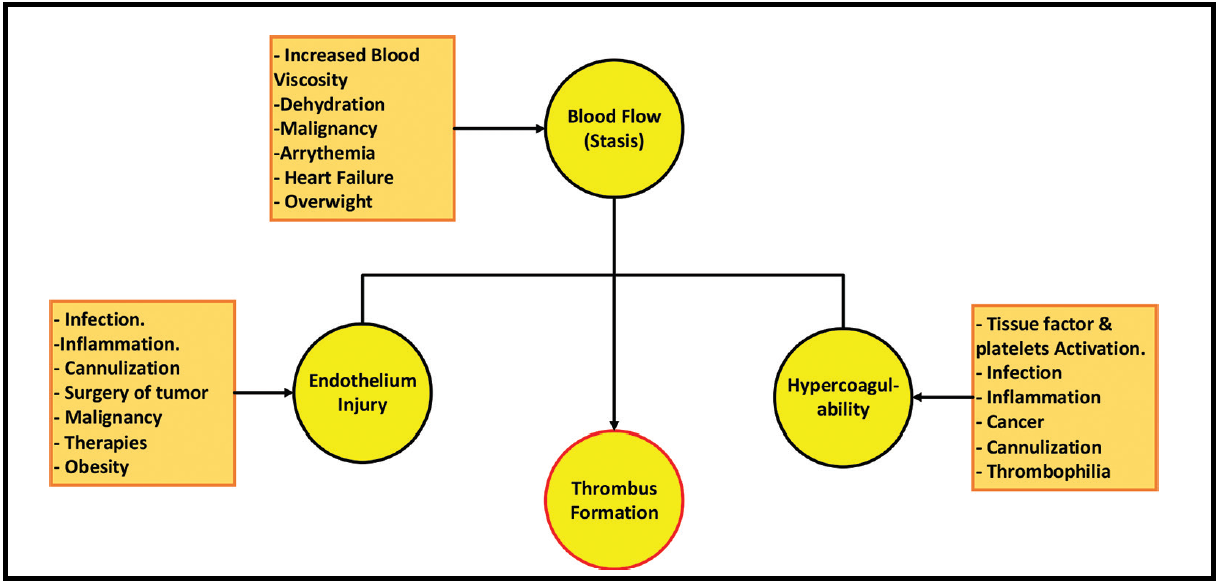
Figure 1: Pathogenesis of thrombus formation
Venous Thromboembolism Risks and Chemotherapy
To prevent VTE, it is crucial to identify high-risk cancer patients who might benefit from thromboprophylaxis. The prevalence of symptomatic VTE ranging from 5% - 7% is undoubtedly comparable to or even higher than that observed in hospitalized or postoperative patients, where VTE prophylaxis has demonstrated remarkable efficacy [51,52]. Risk assessment models have been used clinically to assess DVT and PE risk in other populations [53,54], which do not apply to cancer patients [55]. A prevalent complication of cancer and antineoplastic treatment is the occurrence and recurrence of VTE.
More studies have identified associated risk factors with cancer associated VTE. These factors comprise the primary cancer origin and location, the existence of metastases, and the utilization of antineoplastic treatments such as chemotherapy, hormonal therapy, surgical procedures, and erythropoiesis-stimulating agents [56–58]. Active-treatment cancer patients have the highest VTE development risk. Chemotherapy increased thrombosis risk by 6.5-fold, whereas cancer patients have a 4.1-fold VTE-increased risk, according to a population-based study [59]. Interestingly, it was reported that breast cancer patients have a higher VTE rate, especially stage II, which reduces significantly after chemotherapy completion [60].
Significant ramifications ensue for cancer individuals who develop VTE. These include the need for ongoing anticoagulation, potential chemotherapy delivery delays, a heightened likelihood of recurrent VTE, increased bleeding complications risk while on anticoagulants, diminished life quality, and increased healthcare cost [61]. Furthermore, even after the adjustment to the disease stage, VTE increases mortality by twofold or more compared to patients who had cancer without VTE [62]. Thromboembolism is the principal cause of mortality among cancer patients [23].
In several high-risk populations, including hospitalized patients and those undergoing surgery, primary VTE prophylaxis reduces fatal PE and DVT [63,64]. Effective prophylaxis and cancer patient identification at risk for VTE could lead to improvements in cancer-related morbidity and mortality, cancer therapy delivery, life quality, and savings in healthcare costs. Cancer outpatients who met specific risk factors, including lung cancer, metastatic breast cancer, and intravenous catheter presence have participated in clinical trials involving thromboprophylaxis [65,66]. However, 19 of these studies have been unsuccessful in demonstrating the benefit of VTE primary thromboprophylaxis. Present consensus guidelines do not advise prophylaxis for outpatients with cancer [67].
Furthermore, another important precipitating risk factor that increases VTE in malignancy is the route of chemotherapy administration. Chemotherapy is usually given via a small cannula in the peripheral vein. However, the peripheral veins are small and usually damage quickly due to repeated puncturing and chemotherapy. Hence, central vein catheter (CVC) and peripheral vein catheter (PICC) are inserted and used for chemotherapy. These two modalities make the infusion of chemotherapy agents more accessible and more convenient, with less damage to veins, less oozing risk of chemotherapy, and painless chemotherapy sessions.
Central Venous Catheter Thromboembolism in Cancer
Central and peripherally inserted PICC lines are commonly used to administer chemotherapy in cancer patients. Peripheral PICC lines have a higher rate of thrombosis compared to central venous lines [68]. A CVC is required to enable chemotherapy for various cancer patients. Besides the increased infection rate, arterial canulization, hemorrhage, and hematomas, central venous catheterization is linked with catheter-induced thrombosis and thromboembolism risk, especially in cancer patients [69]. These can lead to complications that significantly promote morbidity and death in cancer individuals [70]. The most common non-infectious consequence linked to central line use is thrombosis, resulting in chemotherapy delay [71]. Central line vein thrombosis ranges between 5% - 18% [72,73]. Another report revealed that catheter-related thrombosis clinical evidence is seen in around 4% - 8% of cancer patients [74].
The average occurrence rate of Catheter-related thrombosis, identified using either echography or Doppler, falls within the range of 12% - 14%, exhibiting a significant negative projecting value of around 95%. Currently, there is no recognized thromboprophylaxis for PICC or CVC thrombosis despite its high occurrence and significant medico-economic impact. Catheter related thrombosis often manifests promptly after catheter placement, mostly during the first 7 days and universally within the initial month post-insertion. To avoid catheter-linked thrombosis, it is imperative to consider various local and systemic risk factors. This may be achieved by ensuring proper catheter insertion and maintenance protocols are followed consistently. Routine recommendations do not support the regular use of primary pharmacological thromboprophylaxis to prevent catheter-linked thrombosis. However, it may be explored in certain instances [71].
Meta-analyses indicate that the incidence of both asymptomatic and symptomatic catheter-linked thrombosis may be reduced by around 55% – 60% when using either VKAs or LMWHs without a significant bleeding risk increase. The efficacy of this type of VTE prophylaxis is contingent upon initiation before the implantation of the central venous catheter, at dosages intended for prophylaxis, and subsequent continuation at doses below therapeutic levels [74], with accepting the risk of bleeding during insertion.
A considerable number of researchers have endeavored to ascertain efficacious catheter-related thrombosis prophylactic regimes. At first glance, an open randomized study indicated that the administration of low-dose warfarin (1 mg/day) might substantially diminish the occurrence of catheter-related thrombosis among cancer patients (from 37.5% to 9.5%) [75]. Nevertheless, several subsequent RCTs have been unable to establish any protective effect associated with fixed low-dose warfarin [65,74,76]. Similary, RCTs utilizing LMWH showed comparable results to low-dose warfarin. In comparison to no treatment, dalteparin 2500 IU single daily dose substantially decreased CVC-related thrombosis, according to a small open RCT (p = 0.002) [77]. Nevertheless, despite subsequent larger RCTs utilizing nadroparin, dalteparin, and enoxaparin DVT prophylactic regimens, there were no reductions in catheter-related thrombosis observed [66,78–80]. A significant diminution in catheter-related thrombosis was observed in pediatric and adults following bone marrow transplant in a small open-label study of continuous infusion unfractionated heparin (p = 0.03) [81]. Lavau-Denes et al. conducted a study involving 420 patients and observed that those treated with a prophylactic dose of VKA or LMWH had a lower catheter-related thrombosis incidence (8.1%) compared to those who had not received any anticoagulation (14.8%) [82].
Anticoagulant CVC thromboprophylaxis has been the subject of meta-analyses [82–85]. As the all-anticoagulant modalities data were combined, Akl et al. observed a 44% relative risk drop in symptomatic CVC-associated thrombosis episodes, whereas other studies had not observed such a drop [85]. A study observed that LMWH and VKA reduced the relative incidence of symptomatic and asymptomatic catheter-related thrombosis by 63% and 28%, respectively [86]. In contrast, others did not find this information, possibly because their inclusion criteria differed [87,88]. A meta-analysis review reported that the relative reduction in the risk of catheter-related thrombosis was observed more with LMWH in symptomatic events (-52%) and exclusively with VKA in asymptomatic events (57%) [86]. There was no evidence of superiority in any antithrombotic regimen (e.g., Vitamin K antagonists versus LMWH) according to any meta-analysis. There were no noted disparities in significant hemorrhage or mortality rates between the control group and the patients who were administered anticoagulants. Therefore, it is not recommended to administer pharmacologic prophylaxis on a routine basis to prevent thrombosis that is associated with central vein catheters [74,89–91]. Although most meta-analyses found prophylactic anticoagulation to be beneficial, few clinical trials observed a reduced catheter-related thrombosis rate. Dejectedly, the findings of different large, randomized studies conducted to date may have two significant limitations. The dosage of the anticoagulant is the initial restriction. Young et al. reported that 1590 patients with malignancy and CVC thrombosis received 1 mg/day warfarin compared to no warfarin. they did not found a significant decline in the catheter-associated thrombosis occurrence rate that was confirmed radiographically as symptomatic (p = 0.98) [76]. However, dose-adjusted warfarin significantly decreased the incidence of such complications (p = 0.002), although the cost of this was an increase in MB complications (p = 0.04). The delayed and highly variable initiation of antithrombotic prophylaxis after catheter insertion constitutes the second constraint. Hence, the potential lack of benefit observed by the authors may be credited to the administration of prophylactic medication after the onset of the pathophysiologic processes that precipitated thrombosis. However, further studies are required to assess the benefit of warfarin in the prevention of CVC thrombosis.
While precise data regarding the pathogeny of catheter-related thrombosis is lacking, it has been underscored that cancer patients face a significantly heightened risk for this complication in the initial hours or days following the insertion of a central venous catheter [78,92]. Catheter insertion appears to be the most significant risk factor for catheter-associated thrombosis [74,89,93–95]. This is likely due to endothelial injury or direct blood vessel wall trauma, which increases thrombus likelihood, especially in the hours or days following the insertion of a central venous catheter when the repair processes are not yet complete [78]. As De Cicco et al. demonstrated, this evidence emphasizes the critical nature of initiating antithrombotic prophylaxis before the insertion of CVC. They studied 348 cancer patients who were assigned to receive either 5000 IU dalteparin or 1 mg acenocoumarin daily, three days and two hours preceding the insertion of the central venous catheter, or no anticoagulant treatment [78]. Following catheter insertion, VKA and LMWH therapy lasted for eight days [78]. The anticoagulated group (on VKA and LMWH) had a lower catheter-related thrombosis incidence on days 8 and 30 after CVC insertion (21.9% and 40.0%, respectively) compared to the observation group (52.6%; p < 0.01). The higher efficacy of acenocumarine in comparison to dalteparin may have been attributed to an overdose of VKA, as 10% of acenocumarine patients group exhibited an INR value greater than 1.5 [78].
The dosages of prophylactic agents utilized in this investigation were deemed safe, as no significant hemorrhaging occurred. Similarly, the administration of antiplatelet agents or anticoagulants before catheter insertion was found to be beneficial in two studies [76,96]. According to the WARP study, warfarin administration could commence three days before the insertion of a central venous catheter to ensure sufficient drug exposure in the immediate postinsertion phase. This factor potentially contributed to the favorable outcomes observed in this trial [74,76]. In the ONCOlogie et Chambres ImPlantables (ONCOCIP) study, 3032 cancer patients were monitored for 12 months [96]. It was observed that patients who had used continuous antiplatelet treatment at the outset had a reduced catheter-related thrombosis incidence [96]. Although there are conflicting reports about the benefit of anticoagulation prior to or after the central vein catheter insertion, it seems that further studies need to clarify whether and when to start anticoagulation and which anticoagulant to give, which needs further research projects.
Anticancer Effects of Anticoagulants
Patients with advanced cancer and metastasis sometimes need to be bedridden for extended periods. This greatly heightens the likelihood of developing VTEs [97]. There has been an increased interest in the potential effects of anticoagulants for treating cancer and complication prevention. This is because there are connections between blood clotting and the biology and prognosis of cancer [98]. Specific angiogenic mechanisms are promoted in cancer development as a result of cellular interactions, localized oxygen deprivation, and the presence of specific cytokines and growth factors [16,98]. It was observed that anticoagulants might decrease 5-fluorouracil effectiveness, highlighting potential flaws in treating VTE in these types of patients [98].
Vitamin K Antagonist Anticancer Effect
VKA use showed antiproliferative properties in pancreatic cancer [99]. In an investigation, Coumarin, a warfarin component, inhibits tumor cell growth and migration [100]. Vitro studies showed anti-adhesive VKA actions and decreased breast cancer metastasis [101]. Human observational studies presented that warfarin reduces the overall incidence of lung, prostate, colon, and breast cancers [102]. Moreover, O'Rorke et al. reported an association between warfarin pre-diagnostic use and cancer-specific mortality in breast, lung, prostate, and colorectal cancer [103]. Warfarin functions as an antiproliferative and antimigratory agent achieved by inhibiting the γ-carboxylation of the Gla-domain on gamma-carboxglutamic acid-6 (Gas6). This leads to reduced activation of receptors and diminished Axl (receptor tyrosine kinase) signaling [99]. Gas6 is a gamma-carboxyglutamic acid (Gla) domain-comprehending protein thought to be involved in the stimulation of cell proliferation. Treatment with Vitamin K antagonists (VKAs) in a preclinical model of pancreatic cancer inhibits the growth of the disease at both its primary and metastatic locations [99]. Inhibition of Gas 6-mediated activation of the Axl receptor tyrosine kinase on cancer cells constitutes the molecular mechanism underlying the antitumor activity, partially unrelated to its impact on coagulation [99].
Heparin Anticancer Effect
Heparin, a heterogeneous blend of glycosaminoglycans, has potent anticoagulant properties. It is extensively used, with its derivatives, as an anticoagulant to avert venous thromboembolism. Fortuitously, during the treatment of cancer patients at risk, it has been discovered that heparin and similar medications have anticancer properties [97]. Heparin and its derivatives impact the growth, attachment, formation of new blood vessels, movement, and infiltration of cancer cells via different mechanisms. The primary mechanisms include the suppression of heparanase, angiogenesis, P-/L-selectin, and CXCL12-CXCR4 axis disruption [98,104,105]. These additional biological activities might contribute to the observed anticancer effects. In vitro studies suggest that heparin and derivatives inhibit epithelial cancer growth, migration, and invasion [106–108]. Pre-treatment of experimental lung metastasis models with LMWHs before intravenous inoculation of cancer cells consistently inhibits metastasis development [105]. Conversely, most research studies fail to demonstrate antitumor effects when employing animal models with pre-existing malignancies [105].
Research has shown that LMWH inhibits the growth of different cancer cell lines, such as melanoma, breast, and colon cancer [109,110]. Camacho-Alonso et al. found that low-molecular-weight heparin reduces oral cavity squamous cell cancer (OCSCC) proliferation [111]. In a synergistic effect with cisplatin, LMWH's antiangiogenic action inhibits thrombin synthesis, tissue factor expression, and fibrin creation, preventing tumor cell proliferation. In the oral cavity, squamous cell and lung adenocarcinoma, enoxaparin inhibits PI3k/Akt and mitogen-activated protein kinase/Extracellular signal-regulated kinase (MAPK/ERK) signaling pathways to diminish tumor growth and migration [106]. The primary function of the PI3K-AKT signaling pathway is cellular apoptosis inhibition while simultaneously stimulating cell proliferation and growth (i.e., controlling the cell cycle).
In addition, enoxaparin suppressed the growth and movement of A549 cells [106]. Enoxaparin caused a decrease in the activity of MAPK and PI3K, resulting in a reduction in the production of matrix-metalloproteinase (MMP-2) and the inhibition of A549 cell migration. The MMP-2 controls chemokine monocyte-chemotactic protein 3 processing, generating a chemokine receptor antagonist [112]. Furthermore, enoxaparin enhanced the effectiveness of doxorubicin by promoting apoptosis without any detectable impact on cell-cycle advancement. The research concluded that enoxaparin's ability to prevent cancer in A549 cells was achieved by disrupting two primary PAR-1 downstream signaling pathways, namely MAPK/ERK and PI3K/Akt. This disruption subsequently hinders cell proliferation and migration [106]. Hence, enoxaparin shows potential as a supplementary treatment to conventional lung cancer chemotherapy and justifies further research.
In patients without malignancy, anticoagulant therapy with LMWHs or VKAs has been effective in preventing recurrent VTE [113]. Research has demonstrated that LMWH treatment is more effective than VKA treatment for reducing recurrent VTE rates in cancer patients. Self-administered LMWHs are unpopular with patients who would rather receive the medication orally, as they necessitate tiresome daily subcutaneous injections. Oral VKAs possess a limited therapeutic window and are vulnerable to numerous drug and dietary interactions [114]. Moreover, regular more frequent monitoring of the anticoagulant effect is necessary [115].
Direct Oral Anticoagulants (DOAD) Anticancer
DOACs are a newly developed category of oral anticoagulants. They accomplish their action by directly binding to and impeding the thrombin activity or factor Xa (i.e., apixaban, rivaroxaban, and edoxaban). Furthermore, the activated factors X and thrombin initiate the coagulation cascade and stimulate the expression of protease-activated receptors on cancerous and healthy cells [116]. Additionally, factor Xa and thrombin activate the intracellular signaling pathways (Ras, PI3K, mTor) that regulate cancer cell adhesion, migration, and patient survival [116]. Moreover, by forming fibrin/platelet-rich aggregates that encircle circulating tumor cells, coagulation can indirectly aid in widespread metastasis [117], protecting against natural killer-cell-mediated assaults and shear stress. Thrombin triggers tumor cell adhesion to endothelial cells, subendothelial matrix protein, and platelets, promoting tumor development and angiogenesis [116]. Circulating active factor X may overexpress sticky receptors in endothelial cells, promoting the spread of cancer [118]. Hence, DOAC agents that prevent factor X activation may slow this mechanism, limiting tumor survival and metastasis.
Dabigatran administration did not affect OCSCC migration [98]. However, breast cancer-type cells were tested with dabigatran showed decreased cell viability [119,120]. Moreover, oral dabigatran lowers breast cancer cell invasiveness in vitro and mouse tumor development and metastasis [121]. In breast and pancreatic cancer animal models, dabigatran synergistically inhibits tumor development and progression with chemotherapeutic treatments [121,122], which was not noticed in OCSCC [98]. Rivaroxaban, apixaban, and edoxaban inhibit H400 and H357 cancer cell proliferation at various doses. Different mouse model studies indicated no antitumorigenic effects from rivaroxaban [123–125]. A mouse-modeled study showed that high-dose apixaban exhibited widespread growth attenuation in colon, prostate, and ovary cancer cell growth [126]. Besides, apixaban reduces ovary and large bowel cancer migration in high-dose therapy [127]. In contrast, rivaroxaban did not impact migration, while low-dose apixaban sped wound closure and high-dose edoxaban at 6-9 hours; however, this effect had an insignificant impact after 12 hours [98].
Anticoagulation in Ambulatory and Hospitalized Cancer Patients
Researchers have shown that VTE prophylaxis effectively lowers the likelihood of VTE, although it can cause serious bleeding [128–130]. Nevertheless, the bulk of these trials were conducted previously and mostly focused on radiologic endpoints rather than clinical ones. The presence of these constraints has led to a reassessment of the universal use of thromboprophylaxis in hospitalized patients. This reappraisal is very pertinent to patients receiving medical oncology treatment while hospitalized since no studies have been undertaken to assess the primary VTE prophylaxis effectiveness in medically sick cancer patients. Additionally, only a few participants in significant medical prophylaxis trials involved cancer patients [15]. Therefore, there is just a small amount of data to support the use of VTE prevention in hospitalized medical oncology patients.
Several VTE risk assessment models (RAMs) have been created for medically unwell or undergoing surgery in hospitalized patients [131]. While some of these RAMs have been confirmed in separate groups of patients, it is not yet appropriate to use these models to evaluate the risk in cancer patients. Every RAM considers all types of cancer to have an equal risk of VTE. Furthermore, none of the models included any additional well-established risk factors for cancer related VTE. None of the RAMs underwent development or validation in cancer patient populations. Hence, it was suggested that all medical and surgical oncology hospitalized patients should be given thromboprophylaxis per the criteria set by the National Comprehensive Cancer Network (NCCN) [132,133].
The American Society of Clinical Oncology (ASCO) recommendations advise the regular use of thromboprophylaxis in all hospitalized patients, except those treated just for a minor operation or chemotherapy [134]. Future research should prioritize showing the effectiveness of VTE prevention in hospitalized oncology patients and creating and verifying cancer specific VTE RAMs.
The problems the cancer patients had with VTE prevention were carefully investigated. A prospective study including 2373 cancer surgery patients found that 2.1% of patients had symptomatic VTE throughout a 30- to 65-day. The average duration until the occurrence of VTE was 17 days. More than 40% of the occurrences occurred more than 21 days after the procedure. The percentage of patients who got thromboprophylaxis during the hospitalization was above 80%, while 31% had prophylaxis after being discharged, and 23% maintained prophylaxis beyond day 21. Being 60 years of age, having a history of VTE, experiencing anesthesia for a minimum of 2 hours, having an advanced-stage malignancy, and being confined to bed rest for 4 days are factors that significantly increase the VTE likelihood in individuals with cancer. Among the whole patient population, 41 individuals experienced death after surgical procedures. Out of the total, 19 fatalities (46%) were attributed to VTE, whereas 4 deaths (10%) were due to hemorrhage. Among the four deceased patients, three had previously discontinued the use of VTE prevention [135]. The results have provided evidence for using an initiative-taking strategy for preventing VTE following surgical therapy for cancer patients.
The Enoxacan II trial showed that prolonging the use of enoxaparin to 21 days after surgery, instead of the customary 6 - 10 days, resulted in a 60% decrease in the VTE relative risk. VTE incidence was 4.8% in the extended-duration enoxaparin group compared to 12% in the standard-duration group. The relative risk reduction was 60% (p-value = 0.02) [136]. Nevertheless, out of the 20 events seen in the placebo group, only 4 were cases of proximal DVT or PE, with just 1 incident reported. A meta-analysis of 7 prospective trials on extended-duration prophylaxis following cancer surgery revealed a 56% reduction in total VTE cases (2.6% vs 5.6%) and a 54% reduction in proximal DVT cases (1.4% vs 2.8%). A small decrease in the PE rate was seen in the extended enoxaparin patients' group, with a rate of 0.8% compared to 1.3%. The MB incidence was 1.8% compared to 1.0%, and the rate of all-cause death was 4.2% compared to 3.6% [137,138]. According to these findings, the NCCN and ASCO recommendations suggest that high-risk patients undergoing abdominal-pelvic cancer surgery should get extended-duration prophylaxis [134,139].
Research conducted by Khorana et al. observed a group of ambulatory cancer patients who were undergoing chemotherapy. They found that 1.9% of these patients had VTE for 2.4 months, as determined by a median follow-up time [57,140]. Following subsequent randomized thromboprophylaxis studies, there have been notable decreases in VTE, but the actual reductions were not very large [141,142]; however, other studies observed significant decreases in VTE risk in advanced pancreatic cancer [143,144].
In multiple myeloma, VTE risk is nine times greater due to the underlying illness and its treatment [145]. In a clinical study that randomly assigned low-risk myeloma patients to receive either aspirin (ASA) 100 mg daily or ENOX 40 mg daily, it was shown that both treatments were equally effective for preventing VTE. VTE incidence was 2.27% in the ASA group compared to 1.20% in the ENOX group [146]. Khorana et al. have devised a risk assessment tool based on evidence to effectively identify cancer patients who are most likely to benefit from prophylaxis. This method has been verified in many independent investigations [147]. Currently, there are ongoing prospective trials that are assessing the effectiveness of DOACs for VTE prevention in high-risk cancer patients.
NCCN and ASCO recommendations advise administering thromboprophylaxis to myeloma patients who are undergoing chemotherapy regimens, including anti-angiogenesis agents. High-risk myeloma patients should have anticoagulant thromboprophylaxis, whereas lower-risk individuals should be given low-dose ASA. The ASCO recommendation suggests that thromboprophylaxis should be considered for additional carefully chosen high-risk individuals. Thromboprophylaxis is not recommended for ambulatory cancer patients and not participating in clinical trials, according to the NCCN guideline [134,139].
MANAGEMENT OF THROMBOEMBOLISM IN CANCER PATIENTS
Recommended Initial, Short, and Long-term Venous Thromboembolism Therapy in Cancer
VTE treatment can be categorized into three phases: the initial phase (which lasts for the first 5 to 10 days of therapy), the short-term phase (which spans from month 1 to month 6), and the long-term phase (which lasts for 6 months). Meta-analyses have shown that there is no discernible disparity in the episode of recurrent thrombosis or bleeding between LMWH and unfractionated heparin [148,149]. Unfractionated heparin is recommended for hospitalized patients who need invasive operations or those who have a significant risk of bleeding. LMWH continues to be the recommended treatment for the first and short-term VTE management in cancer. In the Comparison of Low-molecular-weight heparin versus Oral anticoagulant Therapy (CLOT) trial, which compared LMWH (dalteparin) with oral anticoagulant therapy (warfarin) for preventing recurrent VTE in cancer, dalteparin was found to reduce the occurrence of recurrent VTE by 50% compared to warfarin (dalteparin vs warfarin; 9% vs 17%). Additionally, dalteparin had a comparable MB risk (dalteparin vs warfarin; 6% vs 4%). The mortality rates were comparable between the two groups, with dalteparin at 39% and warfarin at 41% [113].
Comparison of Acute Treatments in Cancer Haemostasis (CATCH) study conducted for 900 patients suffering from active malignancy were randomly assigned to receive either a daily subcutaneous dose of tinzaparin (175 units/Kg body weight) or a transition from tinzaparin to warfarin with an international normalized ratio target range of 2-3. Recurrent VTE incidence was quantitatively lower in patients given tinzaparin compared to those who had warfarin (7.2% vs 10.5%). The incidence of significant bleeding (2.7% for tinzaparin vs. 2.4% for warfarin and death (34.7% for tinzaparin vs 32.2% for warfarin) were comparable [150]. The duration of these trials was restricted to 6 months.
The DALTECAN trial was conducted to examine the results linked to the extended use of LMWH for treating VTE in cancer. The DALTECAN trial recruited patients with active cancer and new VTE to receive dalteparin for 12 months to verify the effectiveness of long-term LMWH. Out of the 334 patients that were recruited, 185 of them (54.7%) successfully finished 6 months of therapy, while 109 patients (32.2%) completed the full 12 months of treatment. The primary causes for withdrawal were mortality (73 cases, 33.2%), adverse events (60 cases, 26.2%), voluntary withdrawal of permission (42 cases, 18.3%), and 37 patients (11.1%) who had recurrent VTE. The recurrent VTE rate was 8.7% during the initial 6 months and 4.1% between months 7 - 12. Thirty-four patients (10.2%) had significant hemorrhage. The occurrence of significant hemorrhage was 3.6% during the first month and ranged from 0.8% - 1.8% per month between months 2 and 6, and from 0% to 1.4% per month between months 7 and 12. Within the 12-month trial period, 116 patients, accounting for 33.8% of the participants, succumbed to their conditions. Specifically, 105 patients died because of cancer, 2 from hemorrhage, and 4 from PE [151].
A review article by Kalhale et al. concluded that long-term VTE treatment in cancer, LMWHs in comparison to VKAs might produce a considerable VTE rate decrease. In contrast, DOACs versus LMWH, DOAC are more effective in the reduction of VTE, albeit they might increase the MB risk. Hence, the risks and advantages of the anticoagulant agents to be used must be carefully assessed in long-term anticoagulation [152]. While systematic reviews and recommendations advocate for using LMWH in treating VTE in cancer, a study of the Registro Informatizado de Enfermedad TromboEmbolica indicates that VKAs might potentially be beneficial for cancer patients who have had 6 months of LMWH medication. Out of the 1502 malignancy patients who were treated with LMWH for 6 months, 763 patients chose to remain with LMWH, whereas 739 patients opted to switch to warfarin. The VTE recurrent incidence and serious bleeding events were comparable [153]. An analysis of malignancy patients with VTE found that warfarin treatment had more positive outcomes when compared to LMWH [154]. These trials indicate that VKAs may have comparable efficacy to LMWH in a substantial number of malignancy patients with VTE. Additional investigation is necessary to validate these results.
Extensive randomized clinical studies have shown that DOACs are as efficacious, if not more so, compared to traditional treatment including LMWH followed by VKA. Nevertheless, a limited cohort of cancer patients were included in different trials. Vedovati and colleagues conducted a meta-analysis and observed that recurrent VTE occurred in 3.9% of DOAC-treated patients, compared to 6% of VKA-utilized patients. Additionally, severe bleeding occurred in 3.2% of DOAC patients and 4.2% of VKA patients [155]. The data of these trials demonstrate a considerable improvement compared to prior research on cancer-related blood clotting. This could indicate that the patients included in the DOAC studies were not similar to the patients included in the CLOT and CATCH studies [113,150,155–157].
Within the RECOVER investigation, a mere 13% of patients who had active cancer at the beginning of the study had developed metastatic illness. Additionally, the overall death rate for all causes was just 15% [156]. Prins et al. reported that the proportion of cancer patients at the beginning of the study who had metastatic illness was only 22%, and 30% of them had chemotherapy as a form of treatment. The overall death rate was just 16% [158]. A study by Agnelli et al. noted that over one-third of participants had metastatic illness. However, the death rate for cancer patients receiving apixaban was 6%, whereas for those taking warfarin, it was 7.7% [159]. Moreover, there were notable discrepancies in the criteria for inclusion and exclusion across the studies on DOACs in CLOT and CATCH studies.
A total of 200 cancer related VTE patients received a complete course of rivaroxaban. Symptomatic proximal lower limb DVT, recent or recurrent PE, clinically significant non-MB, MB, leading to rivaroxaban cessation, or death were the key outcomes at 6 months in competing risk analysis. The 6-month new or recurrent VTE cumulative incidence was 4.4%, severe bleeding was 2.2%, and all-cause death was 17.6% in competing risk analysis. In this research, new or recurrent VTE and serious bleeding rates were similar to the EINSTEIN cancer subgroup analysis [157]. A study suggested that Rivaroxaban is safe and effective for cancer-related VTE, compared to LMWH [160].
Another previous Research conducted that involved 118 patients with cancer-linked thrombosis who were treated with rivaroxaban saw a non-significant difference in VTE recurrence in cancer and noncancerous patients (3.3% versus 2.8%, respectively) after an average follow-up period of 1.3 years [158]. Chemotherapy was administered to 90 cancer patients, accounting for 76% of the total. Out of the total number of patients, specifically, 22% of those who had cancer passed away. However, none of the non-malignant patients died. Comparable results were documented in research conducted at the Memorial Sloan Kettering Cancer Center [158]. Despite the promising nature of these findings, the author maintains that it is early to use DOACs as primary agents for treating cancer related VTE. The Hokusai VTE Cancer Study and similar ongoing studies will be crucial in validating the findings of previous trials. Therefore, the NCCN and ASCO recommendations advise against DOAC use until more data is available about their effectiveness in individuals with cancer.
The current recommendations from NCCN, ASCO, and the American College of Chest Physicians advise that anticoagulation better be maintained for cancer-associated VTE as long as the cancer is not cured or continuing cancer treatment [15,139,161]. There is insufficient data from randomized studies to determine the optimal duration for anticoagulation therapy in cancer patients. Therefore, the only sources of advice are professional opinion and common sense. Hence, medical practitioners should evaluate these options individually by examining unbiased imaging results for enduring malignancy, tumor markers, and enduring risk factors for VTE, together with the patient's preferences, the likelihood of bleeding associated with anticoagulation, and treatment adherence.
Several risk factors and reasons for recurrent VTE have been found in cancer, including a validated RAM called The Ottawa Score [162]. The Cancer Duration of Anticoagulation based on Compression UltraSonography (DACUS) study found that a negative duplex ultrasound after therapy is associated with a significantly decreased recurrent VTE risk. Specifically, the risk was 2.8% for those with a negative ultrasound compared to 21.9% for those without. Furthermore, end-of-therapy duplex tests provide evidence of any remaining illness if the patient has symptoms that might indicate a new blood clotting event. Despite the varied outcomes of validation studies on the Ottawa Score, the authors believed it is vital to consider established evidence-based risk factors when deciding on anticoagulant medication for cancer patients [163,164]. Despite all these recommendations, there are still ambiguities in deciding when to start and when to stop anticoagulation in malignancy patients to prevent this highly prevalence complication. Therefore, further trials are required to establish a plan for cancer patients concerning VTE prevention.
Venous Thromboembolism Therapy in Thrombocytopenic Cancer Patients
A 50 x 109/litter thrombocyte count is widely recognized as the minimum safe level for therapeutic anticoagulation [134]. According to the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) VTE prophylaxis registry, individuals with a thrombocyte count below 50 x 109/L had a threefold increased risk of developing bleeding [165]. Preliminary studies have shown that individuals with thrombocyte counts below 50 x 109/L may benefit from lower doses of anticoagulation [166,167]. The International Society on Thrombosis and Hemostasis has released a recommendation sheet that provides a logical strategy for managing patients with both thrombocytopenia and VTE based on the currently available evidence [168]. Additional research is necessary to assess the effectiveness and safety of conventional and reduced-dose anticoagulation treatments at various thrombocyte counts in cancer patients who develop VTE.
RECURRENT VENOUS THROMBOEMBOLISM MANAGEMENT IN CANCER
Medical therapy
It is crucial to empirically confirm the presence of VTE recurrence because symptoms may be resembled by infections (e.g., pneumonia), cardiac and pulmonary failure, post-thrombotic syndrome, or hepatic or renal disease. The cause or causes of a recurrent VTE has/have to be determined, and an alternative course of treatment should be initiated upon confirmation of the recurrence of the VTE. Alternate treatment options include modifying the dosage or administration schedule, transitioning to fondaparinux (chemically related to LMWHs), which possesses a prolonged elimination half-life, or utilizing an LMWH or unfractionated heparin.
Subtherapeutic anticoagulation resulting from non-compliance with treatment, hemorrhage, or thrombocytopenia are prevalent factors contributing to recurrent VTE. In such situations, it is critical to implement adherence-enhancing strategies (e.g., closer monitoring, perioperative bridging anticoagulation, and alternative anticoagulant regimes). Trousseau syndrome associated with progressive cancer or inadequately managed neoplastic disorders (e.g., persistent erythrocytosis, polycythemia vera) may give rise to intrinsic or endogenous therapeutic resistance. Trousseau syndrome is frequently associated with recurrent or spontaneous deep venous thromboses or migratory superficial in occult or newly diagnosed visceral malignant disease. However, the term is occasionally applied to describe hypercoagulability that is linked to any malignant condition [169].
Heparin therapy and active management of the underlying cancer are critical in preventing recurrent thromboembolism in Trousseau syndrome patients. Effective management of erythrocytosis is vital for the efficacy of anticoagulant regimens in patients with polycythemia rubra vera. In cancer patients, heparin-induced thrombocytopenia may occasionally result in recurrent VTE, requiring the standard therapy for heparin-induced thrombocytopenia. Therapeutic resistance can be induced by extrinsic factors such as vascular stasis caused by compression due to tumor or nodal masses or thrombosis precipitated by turbulence and obstruction of blood flow caused by CVCs or VCFs. In these situations, it is critical to eliminate the source of the stasis.
As a successful strategy for anticoagulation management, LMWH dose escalation has been demonstrated effective in VTE recurrence prevention [170]. Approximately 73% of the 212 cancer patients with recurrent VTE in an international registry had metastatic cancer during the event, and 70% were taking LMWH. Approximately 70% of those with breakthrough thrombosis were receiving a therapeutic or more than therapeutic dose. Following this, 24% of the participants shifted to a different medication, while 31% were prescribed an increased therapeutic dose and 33% continued on the same dosage. Approximately 11% experienced a recurrence of a VTE within three months of follow-up, 8% developed severe hemorrhage, and 27% passed away. Recurrent VTE was a less frequent complication of LMWH compared to VKA [171]. This study highlighted the difficulties and provided support for LMWH use in managing cancer patients with recurrent VTE. However, new prospective studies are required to clarify those issues.
Interventional (Mechanical) Therapy
The Vena cava filter is used to prevent thrombus migration beyond the filter that is usually inserted in the abdominal inferior vena cava. The only indication for a vena cava filter (VCF) in cancer subjects is the presence of an acute DVT and/or PE, together with a contraindication for anticoagulation [172,173]. Given that the likelihood of a thrombotic recurrence occurs during the first month of cancer diagnosis, healthcare professionals will generally deem it suitable to employ a VCF if a patient must stop taking anticoagulant medication during this period. In cases where a patient has experienced a thrombotic event over a month ago, the use of VCFs is considered individually. Factors such as the location and size of the thrombus, the anticoagulation treatment duration, the expected duration of time without anticoagulation, and the patient's other existing health conditions should be considered. Utilizing a retrievable filter is advisable since it maintains the possibility of subsequent extraction. It is essential to establish a methodical monitoring procedure to guarantee the retrieval of filters whenever they become unnecessary [174].
comparison between the effectiveness of available anticoagulants
Cancer-associated VTE has been significantly more effectively treated in recent years. In individuals with cancer, DOACs have become the VTE standard therapy. RCTs comparing the DOACs and LMWHs' safety and efficacy supported their application [175–178]. Despite the demonstrated DOACs' efficacy in reducing the recurrence of thrombosis rate among cancer patients, the potential for hemorrhage remains a significant concern, particularly for individuals with GI malignancies. Hokusai VTE Cancer trial reported that GI cancer patients who received edoxaban treatment experienced severe bleeding events at a substantially significantly higher rate (13.2% vs 2.4%) than those who received dalteparin [175]. With esophageal or gastroesophageal malignancies, patients who were administered rivaroxaban in the SELECT-D study exhibited a significantly higher hemorrhage incidence (36% vs 5%) compared to those who were treated with dalteparin. As a result, patient recruitment for the ongoing trial involving this form of tumor was halted [176]. In contrast, the Apixaban and dalteparin in active malignancy-associated venous thromboembolism (ADAM-VTE) and Caravaggio trials found the bleeding attack incidence did not differ significantly in active malignancy-associated VTE between dalteparin and apixaban participants [177,178], particularly in GI malignancies.
During the early 2000s, research demonstrated that administering a twice-daily oral dose of ximelagatran effectively reduces recurrent VTE [179], averted postoperative VTE, and was a viable immediate treatment option for DVT [180]. However, hepatotoxicity, on the other hand, led to ximelagatran withdrawal [181]. It was reported that DOACs have more significant predictable pharmacokinetic and pharmacodynamic properties than VKAs [182,183]. Randomized clinical studies reported that other DOACs (rivaroxaban, edoxaban, etexilate, apixaban, dabigatran) are the best alternatives to VKA in patients without malignancy [159,184]. These alternatives have comparable or superior antithrombotic efficacy. Rivaroxaban [176] and edoxaban [185] have proven comparable efficacy to dalteparin in cancer patients concerning the composite outcome of significant hemorrhage or recurrent venous thromboembolism. Currently, edoxaban or rivaroxaban is the DOAC of choice for preventing recurrent VTE in cancer-affected persons with a significant risk of hemorrhage [186,187].
Patients diagnosed with GI cancer have an elevated susceptibility to VTE. Based on the results of randomized clinical trials involving cancer-associated VTE, DOACs administered to patients with GI cancer exhibited comparable or superior efficacy but a heterogeneous safety profile [188]. The available data suggests that utilizing DOACs to treat GI cancer-associated thrombosis may increase the clinically relevant non-major bleeding (CRNMB) risk. There were no considerable differences in the MB risks; however, it has been observed recently that DOACs' efficacy in VTE recurrence prevention is comparable to LMWH's efficacy [189].
DOACs have emerged as a viable substitute for LMWHs in managing cancer-induced thrombosis. Although DOACs were found to be comparable to LMWHs in preventing recurrent VTE among genitourinary and GI tract malignancies, subgroup analyses revealed that specific DOACs posed greater risks of hemorrhage than LMWHs [190,191]. However, a systematic review with meta-analysis of DOAC use in acute VTE treatment in GI cancers is required. Severe hemorrhage or recurrent VTE major differences between DOACs and LMWH participants were not noted. Furthermore, comparing luminal and non-luminal GI malignancies revealed comparable levels of significant hemorrhage. On the contrary, the DOAC-treated participants exhibited an increased incidence of CRNMB compared to the LMWH-treated participants.
A study reported that rivaroxaban treatment in atrial fibrillation has a lower rate of causing GI bleeding than warfarin-managed patients [192]. Furthermore, CRNMB and MB in the GI were substantially less within LMWH than among the rivaroxaban-treated group [176]. Cancer patients who received edoxaban in the Hokusai VTE Cancer trial experienced a greater incidence of significant bleeding, but not CRNMB, compared to those who received dalteparin. Also, they observed an increased incidence of GI hemorrhage among GI malignancy patients [193]. In contrast, severe GI hemorrhage risk did not differ significantly between cancer apixaban-treated and LMWH-treated patients [177,178]. Intriguingly, DOAC type was used to treat acute VTE, and bleeding risk analysis did not identify any significant differences between the subgroups treated with apixaban and rivaroxaban in terms of severe bleeding. This finding may advocate that GI cancer therapy of associated VTEs by DOACs is not the only hemorrhage risk factor; however, the CRNMB of rivaroxaban patients was greater in this meta-analysis than LMWH patients.
DOAC users experienced comparable severe bleeding events [194,195] as those taking LMWH despite higher CRNMB [191]. While the present meta-analysis did not identify any statistically significant disparity in the MB rates between patients who used DOACs or LMWHs, a discernible pattern indicated an elevated incidence of MB within the DOAC-treated group. Additionally, the effectiveness of DOACs in VTEs' recurrence prevention was comparable to LMWH. Hence, DOACs ought to be regarded as a viable substitute for LMWH in acute VTE management, as there is no statistically significant disparity in severe hemorrhage among patients with GI malignancies. DOACs relate to an increased CRNMB; this should be fully considered when deciding whether to prescribe them to GI cancer patients. Before beginning treatment, the risk of hemorrhaging should be exposed and discussed clearly with the patient.
A meta-analysis was conducted to compare recurrent VTE risk and overall hemorrhage in cancer-associated thrombosis managed with DAOCs versus LMWH in 3 observational studies [196]. Another meta-analysis of 11 studies involving GI cancer patients was conducted [189]. The visual examination of forest sites was employed to assess the consistency of these studies; the presence of low I2 values indicated the absence or minimal heterogeneity. Rungjirajittranon et al. claimed the inconsistencies are due to the inadequate number of occurrences and patients enrolled, which may hinder the capability to identify statistically significant variations in certain outcome determinations, such as VTE recurrence rate [189]. Additionally, crucial baseline characteristics of the patient that could potentially impact the likelihood of thrombosis were not captured, including patient status (i.e., managed as inpatient or outpatient), sex, age, and cancer treatment.
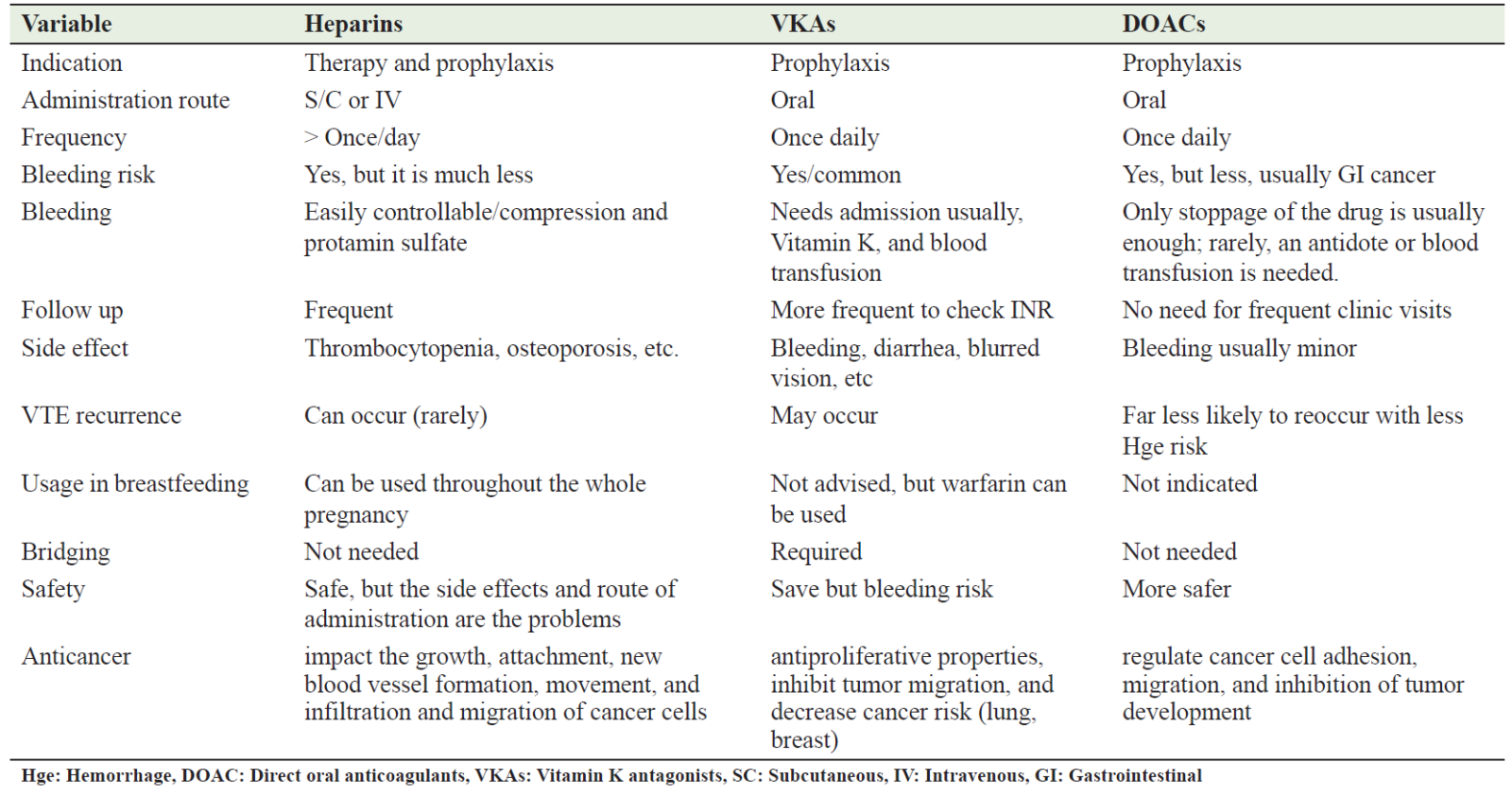
Furthermore, there was variation in the definitions of primary outcomes across the included studies. Fourthly, recurrent thrombosis was the primary outcome in only three studies. Moreover, assessing heterogeneity through analytical means was not feasible due to the meta-analysis's restricted sample size. Lastly, the restricted quantity of studies hindered the assessment of publication bias. Therefore, further prospective studies and meta-analyses based on new data are required to evaluate the best anticoagulant that has a preventive VTE effect and anticancer action in cancerous patients. Table 2 summarizes the comparative differences and side effects between DOAC, heparins, and VKAs in cancer patients. Table 2 compare between the effectiveness of heparin, vitamin k antagonist, , and direct oral anticoagulants medications.
Table 2: Comparison between heparin, VKA, and DOAC medications in cancer patients
Conclusion
VTE occurs frequently as a complication in cancer patients who are actively ill. Over the past two decades, observational studies and randomized clinical trials have made significant contributions to our knowledge of the pathogenesis and treatment of VTE. Nonetheless, there are still unanswered inquiries that require attention. These include the most effective strategy for primary prophylaxis in inpatient and ambulatory patients like medical oncology, the management of cancer associated VTE in populations with standard and high bleeding risks (e.g., appropriate therapy duration, anticoagulant regimen), and the function of DOACs in localized and metastasized cancers. Fortunately, clinical research is actively focused on cancer-associated thrombosis; thus, answers to these crucial concerns should be forthcoming.
Anticoagulation is crucial at the time of diagnosis, after cancer surgery, and during chemotherapy for varied reasons, including the prevention of VTE and its antitumor effects. Nevertheless, the potential for bleeding, tumor dissemination, and VTE recurrence should be carefully considered concerning their advantages. While heparin preparations are considered the safest anticoagulants, their delivery methods may be uncomfortable. VKAs and DOACs are superior due to their oral administration route. Unfortunately, VKAs need regular INR monitoring and have the potential to induce significant and protracted bleeding as compared to DOACs.
AUTHORS’ CONTRIBUTIONS
All authors have made a significant contribution to the work reported, whether in conception, study design, implementation, data collection, data analysis, and interpretation, or all of these areas, they also participated in drafting, revising, or critically reviewing the article and gave final approval to publish the version.
References
- Ashorobi D, Ameer MA, Fernandez R. Thrombosis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538430/. Accessed, Feb 2024.
- Ge SQ, Tao X, Cai LS, et al. Associations of hormonal contraceptives and infertility medications on the risk of venous thromboembolism, ischemic stroke, and cardiovascular disease in women. J. Investig. Med. 2019;67:729–735.
- Hotoleanu C. Association between obesity and venous thromboembolism. Med Pharm Rep. 2020;93(2):162-168.
- Mulder FI, Horváth-Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137(14):1959-1969.
- Lutsey PL, Zakai NA. Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol. 2023;20(4):248-262.
- Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):3-14.
- Lutsey PL, Virnig BA, Durham SB, et al. Correlates and consequences of venous thromboembolism: The Iowa Women’s Health Study. Am J Public Health. 2010;100(8):1506-13.
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54(3):1901647.
- Sud R, Khorana AA. Cancer-associated thrombosis: risk factors, candidate biomarkers and a risk model. Thromb Res. 2009;123(4): 18-21.
- Walker AJ, Card TR, West J, et al. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404–13.
- Mahajan A, Brunson A, White R, Wun T. The Epidemiology of cancer-associated venous thromboembolism: An update. Semin Thromb Hemost. 2019;45(4):321–5.
- Kok VC. Bidirectional risk between venous thromboembolism and cancer in East Asian patients: synthesis of evidence from recent population-based epidemiological studies. Cancer Manag Res. 2017;9:751-9.
- Sogaard KK, Schmidt M, Pedersen L, et al. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation. 2014;130:829–836.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715-22.
- Streiff MB. Thrombosis in the setting of cancer. Hematology Am Soc Hematol Educ Program. 2016;2016(1):196-205.
- Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers (Basel). 2018;10(10):380.
- Kwaan, H.C. Coagulation in Cancer; Kwaan, H.C., Green, D., Eds.; Springer: Boston, MA, USA, 2009.
- Fuentes HE, Tafur AJ, Caprini JA. Cancer-associated thrombosis. Dis Mon. 2016;62(5):121-58.
- Poénou G, Tolédano E, Helfer H, et al. Assessment of bleeding risk in cancer patients treated with anticoagulants for venous thromboembolic events. Front Cardiovasc Med. 2023;10:1132156.
- Singh R, Sousou T, Mohile S, et al. High rates of symptomatic and incidental thromboembolic events in gastrointestinal cancer patients. J Thromb Haemost. 2010;8(8):1879-81.
- Sigrid K, Brækkan, John-Bjarne Hansen. VTE epidemiology and challenges for VTE prevention at the population level. Thrombosis Update. 2023;10:100132.
- Waheed SM, Kudaravalli P, Hotwagner DT. Deep Vein Thrombosis. [Updated 2023 Jan 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507708/.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007 Mar;5(3):632-4.
- Timp JF, Braekkan SK, Versteeg HH, et al. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-23.
- Pavlovic D, Niciforovic D, Markovic M, et al. Cancer-Associated Thrombosis: Epidemiology, Pathophysiological Mechanisms, Treatment, and Risk Assessment. Clin Med Insights Oncol. 2023;17:11795549231220297.
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275.
- Khan F, Rahman A, Carrier M, et al. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: systematic review and meta-analysis. BMJ. 2019;366:l4363.
- Walker AJ, West J, Card TR, et al. When are breast cancer patients at highest risk of venous thromboembolism? A cohort study using English health care data. Blood. 2016;127(7):849-57.
- Xu X, Chlebowski RT, Shi J, Barac A, Haque R. Aromatase inhibitor and tamoxifen use and the risk of venous thromboembolism in breast cancer survivors. Breast Cancer Res Treat. 2019;174(3):785-794.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-53.
- Ahlbrecht J, Dickmann B, Ay C, Dunkler D, et al. Tumor grade is associated with venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2012 Nov 1;30(31):3870-5.
- Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24(7):1112-8.
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90(3):446-55.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24(3):484-90.
- De Stefano V, Za T, Rossi E. Venous thromboembolism in multiple myeloma. Semin Thromb Hemost. 2014;40(3):338-47.
- Hurwitz HI, Saltz LB, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol. 2011;29(13):1757-64.
- Sonpavde G, Je Y, Schutz F, et al. Venous thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis of randomized clinical trials. 2013;87(1):80-9.
- Petrelli F, Cabiddu M, Borgonovo K, Barni S. Risk of venous and arterial thromboembolic events associated with anti-EGFR agents: a meta-analysis of randomized clinical trials. Ann Oncol. 2012;23(7):1672-9.
- Murphrey MB, Quaim L, Rahimi N, et al. Biochemistry, Epidermal Growth Factor Receptor. [Updated 2023 Dec 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482459/. .
- Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708-14.
- Ashrani AA, Gullerud RE, Petterson TM, et al. Risk factors for incident venous thromboembolism in active cancer patients: A population based case-control study. Thromb Res. 2016;139:29-37.
- Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339-46.
- Mosevoll KA, Johansen S, Wendelbo Ø, Nepstad I, et al. Cytokines, Adhesion Molecules, and Matrix Metalloproteases as Predisposing, Diagnostic, and Prognostic Factors in Venous Thrombosis. Front Med (Lausanne). 2018;5:147.
- Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(8):1777-83.
- Aird WC. Vascular bed-specific thrombosis. J Thromb Haemost. 2007;5(1):283-91.
- Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population-based cohortstudy using multiple linked UK electronic health records databases. Lancet. 2019; 394(10203):1041-54.
- Lange SA, Reinecke H. Coronary Artery Disease and Cancer: Treatment and Prognosis Regarding Gender Differences. Cancers (Basel). 2022;14(2):434.
- Velders MA, Hagström E, James SK. Temporal Trends in the Prevalence of Cancer and Its Impact on Outcome in Patients with First Myocardial Infarction: A Nationwide Study. J. Am. Heart Assoc. 2020;9:e014383.
- Rohrmann S, Witassek F, Erne P, et al. Treatment of patients with myocardial infarction depends on history of cancer. Eur. Heart J. Acute Cardiovasc. Care. 2018;7:639-645.
- Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009;122(1):3-14.
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006 Feb 11;332(7537):325-9.
- Leizorovicz A, Cohen AT, Turpie AG, et al.Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004 Aug 17;110(7):874-9.
- Samama MM, Dahl OE, Quinlan DJ, et al. Quantification of risk factors for venous thromboembolism: a preliminary study for the development of a risk assessment tool. Haematologica. 2003;88:1410–21.
- Heinemann LA, Dominh T, Assmann A, Schramm W, Schürmann R, Hilpert J, et al. VTE Risk assessment - a prognostic Model: BATER Cohort Study of young women. Thromb J. 2005 Apr 18;3(1):5.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-7.
- Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost. 2002;87:575–9.
- Khorana AA, Francis CW, Culakova E, et al. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822-9.104(12):2822-9.
- Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715-22.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., III Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000; 160(6):809-15.
- Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J Clin Oncol. 1991;9(2):286-94.
- Elting LS, Escalante CP, Cooksley C, Avritscher EB, Kurtin D, Hamblin L, Khosla SG, Rivera E. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164(15):1653-61.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-64.
- Halkin H, Goldberg J, Modan M, Modan B. Reduction of mortality in general medical in-patients by low-dose heparin prophylaxis. Ann Intern Med. 1982;96(5):561-5.
- Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J . 1988;318(18):1162-73.
- Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23(18):4063-9.
- Verso M, Agnelli G, Bertoglio S, et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double-blind, placebo-controlled, randomized study in cancer patients. J Clin Oncol. 2005;23(18):4057-62.
- Wagman LD, Baird MF, Bennett CL, et al. Venous thromboembolic disease: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4(9):838-69.
- Refaei M, Fernandes B, Brandwein J, et al. Incidence of catheter-related thrombosis in acute leukemia patients: a comparative, retrospective study of the safety of peripherally inserted vs. centrally inserted central venous catheters. Ann Hematol. 2016; 95(12):2057-2064.
- Marin A, Bull L, Kinzie M, et al. Central catheter-associated deep vein thrombosis in cancer: clinical course, prophylaxis, treatment. BMJ Support Palliat Care. 2021;11(4):371-380.
- Haggstrom L, Parmar G, Brungs D. Central Venous Catheter Thrombosis in Cancer: A Multi-Centre Retrospective Study Investigating Risk Factors and Contemporary Trends in Management. Clin Med Insights Oncol. 2020;14:1179554920953097.
- Laguna JC, Cooksley T, Ahn S, et al. Catheter-related thrombosis (CRT) in patients with solid tumors: a narrative review and clinical guidance for daily care. Support Care Cancer. 2022;30(10):8577-8588.
- Lee AY, Kamphuisen PW. Epidemiology and prevention of catheter-related thrombosis in patients with cancer. J Thromb Haemost. 2012;10(8):1491-9.
- Chen Y, Chen H, Yang J, et al. Patterns and risk factors of peripherally inserted central venous catheter-related symptomatic thrombosis events in patients with malignant tumors receiving chemotherapy. J Vasc Surg Venous Lymphat Disord. 2020.
- Debourdeau P, Lamblin A, Debourdeau T, et al. Venous thromboembolism associated with central venous catheters in patients with cancer: From pathophysiology to thromboprophylaxis, areas for future studies. J Thromb Haemost. 2021;19(11):2659-73.
- Bern MM, Lokich JJ, Wallach SR, et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112(6):423-8.
- Young AM, Billingham LJ, Begum G, et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet. 2009;373(9663):567-74.
- Monreal M, Alastrue A, Rull M, et al. Upper extremity deep venous thrombosis in cancer patients with venous access devices--prophylaxis with a low molecular weight heparin (Fragmin). Thromb Haemost. 1996;75(2):251-3.
- De Cicco M, Matovic M, Balestreri L, et al. Early and short-term acenocumarine or dalteparin for the prevention of central vein catheter-related thrombosis in cancer patients: a randomized controlled study based on serial venographies. Ann Oncol. 2009; 20(12):1936-42.
- Karthaus M, Kretzschmar A, Kroning H, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol. 2006; Ann Oncol. 2006 Feb;17(2):289-96.
- Niers TM, Di Nisio M, Klerk CP, et al. Prevention of catheter-related venous thrombosis with nadroparin in patients receiving chemotherapy for hematologic malignancies: a randomized, placebo-controlled study. J Thromb Haemost. 2007;5(9):1878-82.
- Abdelkefi A, Ben Othman T, Kammoun L, et al. prevention of central venous line-related thrombosis by continuous infusion of low-dose unfractionated heparin, in patients with haemato-oncological disease. A randomized controlled trial. Thromb Haemost. 2004;92(3):654-61.
- Lavau-Denes S, Lacroix P, Maubon A, et al. Prophylaxis of catheter-related deep vein thrombosis in cancer patients with low-dose warfarin, low molecular weight heparin, or control: a randomized, controlled, phase III study. Cancer Chemother Pharmacol. Cancer Chemother Pharmacol. 2013;72(1):65-73.
- Kirkpatrick A, Rathbun S, Whitsett T, Raskob G. Prevention of central venous catheter-associated thrombosis: a meta-analysis. Am J Med. 2007;120(10):901.e1-13.
- Akl EA, Ramly EP, Kahale LA, et al. Anticoagulation for people with cancer and central venous catheters. Cochrane Database Syst Rev. 2014(10):CD006468.
- Carrier M, Tay J, Fergusson D, Wells PS. Thromboprophylaxis for catheter-related thrombosis in patients with cancer: a systematic review of the randomized, controlled trials. J Thromb Haemost. 2007;5(12):2552-4.
- Akl EA, Karmath G, Yosuico V, et al. Anticoagulation for thrombosis prophylaxis in cancer patients with central venous catheters. Cochrane Database Syst Rev. 2007(3):CD006468.
- D’Ambrosio L, Aglietta M, Grignani G. Anticoagulation for central venous catheters in patients with cancer. N Engl J Med. 2014;371(14):1362-3.
- Chaukiyal P, Nautiyal A, Radhakrishnan S, et al. Thromboprophylaxis in cancer patients with central venous catheters. A systematic review and meta-analysis. Thromb Haemost. 2008;99(1):38-43.
- Zwicker JI, Connolly G, Carrier M, Kamphuisen PW, Lee AY. Catheter-associated deep vein thrombosis of the upper extremity in cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12(5):796-800.
- Sousa B, Furlanetto J, Hutka M, et al. Central venous access in oncology: ESMO clinical practice guidelines. Ann Oncol. 2015;26(5):v152-v168.
- Schiffer CA, Mangu PB, Wade JC, et al. Central venous catheter care for the patient with cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2013;31(10):1357-1370.
- Tolar B, Gould JR. The timing and sequence of multiple device-related complications in patients with long-term indwelling Groshong catheters. Cancer. 1996;78(6):1308-1313.
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665-3675.
- Liang YJ, Tang LQ, Sun XS, et al. Symptomatic venous thromboembolism associated with peripherally inserted central catheters predicts a worse survival in nasopharyngeal carcinoma: results of a large cohort, propensity score-matched analysis. BMC Cancer. 2018;18(1):1297.
- Mino JS, Gutnick JR, Monteiro R, et al. Line-associated thrombosis as the major cause of hospital-acquired deep vein thromboses: an analysis from National Surgical Quality Improvement Program data and a call to reassess prophylaxis strategies. Am J Surg. 2014;208(1):45-9.
- Decousus H, Bourmaud A, Fournel P, et al. Cancer-associated thrombosis in patients with implanted ports: a prospective multicenter French cohort study (ONCOCIP). Blood. 2018;132(7):707-16.
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14(1):118-128.
- Ling LR, Lin Z, Paolini R, Farah CS, et al. Commonly Prescribed Anticoagulants Exert Anticancer Effects in Oral Squamous Cell Carcinoma Cells In Vitro. Biology (Basel). 2022;11(4):596.
- Kirane A, Ludwig KF, Sorrelle N, et al. Warfarin Blocks Gas6-Mediated Axl Activation Required for Pancreatic Cancer Epithelial Plasticity and Metastasis. Cancer Res. 2015;75(18):3699-705.
- Bai G, Zhao D, Ran X, et al. Novel Hybrids of Podophyllotoxin and Coumarin Inhibit the Growth and Migration of Human Oral Squamous Carcinoma Cells. Front Chem. 2021;8:626075.
- McCulloch P, George WD. Warfarin inhibits metastasis of Mtln3 rat mammary carcinoma without affecting primary tumour growth. Br J Cancer. 1989;59(2):179-83.
- Haaland GS, Falk RS, Straume O, Lorens JB. Association of Warfarin Use With Lower Overall Cancer Incidence Among Patients Older Than 50 Years. JAMA Intern Med. 2017;177(12):1774-80.
- O’Rorke MA, Murray LJ, Hughes CM, et al. The effect of warfarin therapy on breast, colorectal, lung, and prostate cancer survival: a population-based cohort study using the Clinical Practice Research Datalink. Cancer Causes Control. 2015;26(3):355-66. doi.
- Hostettler N, Naggi A, Torri G, et al-selectin- and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. FASEB J. 2007 Nov;21(13):3562-72.
- Borsig L. Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thromb Res. 2010;125(2):66-71.
- Alturkistani A, Ghonem N, Power-Charnitsky VA, et al. Inhibition of PAR-1 Receptor Signaling by Enoxaparin Reduces Cell Proliferation and Migration in A549 Cells. Anticancer Res. 2019;39(10):5297-5310.
- Featherby S, Xiao YP, Ettelaie C, et al. Low molecular weight heparin and direct oral anticoagulants influence tumour formation, growth, invasion and vascularisation by separate mechanisms. Sci Rep. 2019;9(1):6272.
- Mantziou S, Markopoulos G, Thrasyvoulou S, et al. Tinzaparin inhibits VL30 retrotransposition induced by oxidative stress and/or VEGF in HC11 mouse progenitor mammary cells: Association between inhibition of cancer stem cell proliferation and mammosphere .
- Rousseau A, Van Dreden P, Mbemba E, Elalamy I, Larsen A, Gerotziafas GT. Cancer cells BXPC3 and MCF7 differentially reverse the inhibition of thrombin generation by apixaban, fondaparinux and enoxaparin. Thromb Res. 2015 Dec;136(6):1273-9.
- Mousa SA, Linhardt R, Francis JL, et al. Anti-metastatic effect of a non-anticoagulant low-molecular-weight heparin versus the standard low-molecular-weight heparin, enoxaparin. Thromb Haemost. 2006;96(6):816-21.
- Camacho-Alonso F, Gómez-Albentosa T, Oñate-Sánchez RE, et al. In Vitro Study of Synergic Effect of Cisplatin and Low Molecular Weight Heparin on Oral Squamous Cell Carcinoma. Front Oncol. 2020;10:549412.
- Kenny HA, Lengyel E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle. 2009;8(5):683-8.
- Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
- Vranckx P, Valgimigli M, Heidbuchel H. The Significance of Drug-Drug and Drug-Food Interactions of Oral Anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61.
- Najidh S, Versteeg HH, Buijs JT. A systematic review on the effects of direct oral anticoagulants on cancer growth and metastasis in animal models. Thromb Res. 2020;187:18-27.
- Versteeg HH, Heemskerk JW, Levi M, et al. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327-58.
- Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178-85.
- Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10(5):355-62.
- Vianello F, Sambado L, Goss A, Fabris F, et al. Dabigatran antagonizes growth, cell-cycle progression, migration, and endothelial tube formation induced by thrombin in breast and glioblastoma cell lines. Cancer Med. 2016;5(10):2886-2898.
- Buijs JT, Laghmani EH, van den Akker RFP, et al. The direct oral anticoagulants rivaroxaban and dabigatran do not inhibit orthotopic growth and metastasis of human breast cancer in mice. J Thromb Haemost. 2019;17(6):951-963.
- Alexander ET, Minton AR, Hayes CS, et al. Thrombin inhibition and cyclophosphamide synergistically block tumor progression and metastasis. Cancer Biol Ther. 2015;16(12):1802-11.
- Damhofer H, Daalhuisen J, Ten Brink M, et al. Dabigatran potentiates gemcitabine-induced growth inhibition of pancreatic cancer in mice. Mol. Med. 2017;23:13–23.
- Buijs JT, Laghmani EH, van den Akker RFP, et al. The direct oral anticoagulants rivaroxaban and dabigatran do not inhibit orthotopic growth and metastasis of human breast cancer in mice. J Thromb Haemost. 2019 Jun;17(6):951-963.
- Maqsood A, Hisada Y, Garratt KB, et al. Rivaroxaban does not affect growth of human pancreatic tumors in mice. J Thromb Haemost. 2019;17(12):2169-2173.
- Graf C, Wilgenbus P, Pagel S, et al. Myeloid cell-synthesized coagulation factor X dampens antitumor immunity. Sci Immunol. 2019;4(39):eaaw8405.
- Alexander MP, Nasr SH, Kurtin PJ, Casey ET, Hernandez LP, Fidler ME, Sethi S, Cornell LD Renal extramedullary hematopoiesis: interstitial and glomerular pathology. Modern Pathol 2015;28(12):1574–1583.
- Guasti L, Squizzato A, Moretto P, Vigetti D, Ageno W, Dentali F, Maresca AM, Campiotti L, Grandi AM, Passi A. In vitro effects of Apixaban on 5 different cancer cell lines. PLoS One. 2017 Oct 12;12(10):e0185035.
- Kahn SR, Lim W, Dunn AS, et al; American College of Chest Physicians. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e195S-e226S.
- Shoji M, Yamashita Y, Ishii M, et al; J-Khorana Registry Investigators. A Predictive Model for Cancer-Associated Thrombosis in Japanese Cancer Patients: Findings from the J-Khorana Registry. TH Open. 2024;8(1):e9-e18.
- Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical P. Chest. 2012 Feb;141(2 Suppl):e227S-e277S.
- Carrier M, Lee AY. Thromboprophylaxis in cancer patients. Semin Thromb Hemost. 2014;40(3):395-400.
- Huang W, Anderson FA, Spencer FA, et al. Risk-assessment models for predicting venous thromboembolism among hospitalized non-surgical patients: a systematic review. J Thromb Thrombolysis. 2013;35(1):67-80.
- Ye F, Stalvey C, Khuddus MA, et al. A systematic review of mobility/immobility in thromboembolism risk assessment models for hospitalized patients. J Thromb Thrombolysis. 2017;44(1):94-103.
- Streiff MB, Holmstrom B, Ashrani A, et al. Cancer-associated venous thromboembolic disease, version 1.2015. J Natl Compr Canc Netw. 2015;13(9): 1079-95.
- Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243(1):89-95.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346(13):975-80.
- Fagarasanu A, Alotaibi GS, Hrimiuc R, Lee AY, Wu C. Role of Extended Thromboprophylaxis After Abdominal and Pelvic Surgery in Cancer Patients: A Systematic Review and Meta-Analysis. Ann Surg Oncol. 2016;23(5):1422-30.
- Rasmussen MS, Jørgensen LN, Wille-Jørgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD004318.
- Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013 Jun 10;31(17):2189-204.
- Wang T, Liu X, Zhu Y, et al. Antithrombotic strategy in cancer patients comorbid with acute coronary syndrome and atrial fibrillation. Front Cardiovasc Med. 2023;10:1325488.
- Gras J. Semuloparin for the prevention of venous thromboembolic events in cancer patients. Drugs Today (Barc). 2012 Jul;48(7):451-7.
- Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10(10):943-9.
- Maraveyas A, Waters J, Roy R, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48(9):1283-92.
- Pelzer U, Opitz B, Deutschinoff G, et al. Efficacy of Prophylactic Low-Molecular Weight Heparin for Ambulatory Patients With Advanced Pancreatic Cancer: Outcomes From the CONKO-004 Trial. J Clin Oncol. 2015;33(18):2028-34.
- Leebeek FW. Update of thrombosis in multiple myeloma. Thromb Res. 2016;140 Suppl 1:S76-80.
- Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012; 119(4):933-9; quiz 1093.
- Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-7.
- Dolovich LR, Ginsberg JS, Douketis JD, et al. A meta-analysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism: examining some unanswered questions regarding location of treatment, product type, and dosing frequency. Arch Intern Med. 2000;160(2):181-8.
- Akl EA, Kahale L, Neumann I, Barba M, et al.Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
- Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015; 314(7):677-86.
- Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN study. J Thromb Haemost. 2015;13(6):1028-35.
- Kahale LA, Hakoum MB, Tsolakian IG, et al. Anticoagulation for the long-term treatment of venous thromboembolism in people with cancer. Cochrane Database Syst Rev. 2018 6(6):CD006650.
- Chai-Adisaksopha C, Iorio A, Crowther MA, et al. Vitamin K Antagonists After 6 Months of Low-Molecular-Weight Heparin in Cancer Patients with Venous Thromboembolism. Am J Med. 2018;131(4):430-437.
- Marshall AL, Campigotto F, Neuberg D, Rowe B, Connors JM. Recurrency of venous thromboembolism in patients with cancer treated with warfarin. Clin Appl Thromb Hemost. 2015;21(7):632-638.
- Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and metaanalysis. Chest. 2015;147(2):475-48.
- Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-7.
- Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomized control trials. Lancet Haematol. 2014;1(1):e37-46.
- Bott-Kitslaar DM, Saadiq RA, McBane RD, Loprinzi CL, et al. Efficacy and Safety of Rivaroxaban in Patients with Venous Thromboembolism and Active Malignancy: A Single-Center Registry. Am J Med. 2016;129(6):615-9.
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808.
- Mantha S, Laube E, Miao Y, et al. Safe and effective use of rivaroxaban for treatment of cancer-associated venous thromboembolic disease: a prospective cohort study. J Thromb Thrombolysis. 2017;43(2):166-171.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315-352.
- Frere C, Crichi B, Wahl C, Lesteven E, et al. The Ottawa Score Performs Poorly to Identify Cancer Patients at High Risk of Recurrent Venous Thromboembolism: Insights from the TROPIQUE Study and Updated Meta-Analysis. J Clin Med. 2022;11(13):3729.
- den Exter PL, Kooiman J, Huisman MV. Validation of the Ottawa prognostic score for the prediction of recurrent venous thromboembolism in patients with cancer-associated thrombosis. J Thromb Haemost. 2013;11(5):998-1000.
- Menapace LA, McCrae KR, Khorana AA. Predictors of recurrent venous thromboembolism and bleeding on anticoagulation. Thromb Res. 2016 ;140(1):93-8.
- Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79.
- Ibrahim RB, Skewes MD, Kuriakose P. “Sailing in troubled waters”: a review of the use of anticoagulation in adult cancer patients with thrombocytopenia. Blood Coagul Fibrinolysis. 2016;27(6):615-30.
- Napolitano M, Saccullo G, Marietta M, et al. Platelet cut-off for anticoagulant therapy in thrombocytopenic patients with blood cancer and venous thromboembolism: an expert consensus. Blood Transfus. 2019;17(3):171-180.
- Carrier M, Khorana AA, Zwicker J, et al. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost. 2013;11(9):1760-5.
- Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110(6):1723-9.
- Carrier M, Le Gal G, Cho R, et al. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7(5):760-5.
- Schulman S, Zondag M, Linkins L, et al. Recurrent venous thromboembolism in anticoagulated patients with cancer: management and short-term prognosis. J Thromb Haemost. 2015;13(6):1010-8.
- Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015;313(16):1627-35.
- Young T, Sriram KB. Vena caval filters for the prevention of pulmonary embolism. Cochrane Database Syst Rev. 2020;10(10):CD006212.
- Khorana AA, Carrier M, Garcia DA, et al. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis. 2016 Jan;41(1):81-91.
- Raskob GE, van Es N, Verhamme P, et al.; Hokusai VTE Cancer Investigators. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018;378(7):615-24.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol. 2018;36(20):2017-2023.
- McBane RD 2nd, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost. 2020;18(2):411–21.
- Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–607.
- Schulman S, Wåhlander K, Lundström T, Clason SB, et al. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med. 2003;349(18):1713-21.
- Eriksson H, Wåhlander K, Gustafsson D, et al. A randomized, controlled, dose-guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. J Thromb Haemost. 2003;1(1):41-7.
- Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol. 2010;(196):407-18.
- Eriksson UG, Bredberg U, Gislén K, et al. Pharmacokinetics and pharmacodynamics of ximelagatran, a novel oral direct thrombin inhibitor, in young healthy male subjects. Eur J Clin Pharmacol. 2003;59(1):35-43.
- Perzborn E, Roehrig S, Straub A, et al. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov. 2011;10(1):61-75.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-52.
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018;378(7):615-624.
- Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019 Oct;20(10):e566-e581.
- Xiang L, Jin S, Yu Y, Wang D, Chen H. Risk of venous thromboembolism in patients undergoing gastric cancer surgery: a systematic review and meta-analysis. BMC Cancer. 2023 Oct 3;23(1):933.
- Ullah F, Song J, Rojas Hernandez CM, et al. Safety and Effectiveness of Direct Oral Anticoagulants for the Treatment of Gastrointestinal Cancer-Associated Venous Thromboembolism. Oncologist. 2023;28(11):e1005-e1016.
- Rungjirajittranon T, Owattanapanich W, Chinthammitr Y, et al. Direct oral anticoagulants versus low-molecular-weight heparins for the treatment of acute venous thromboembolism in patients with gastrointestinal cancer: a systematic review and meta-analysis. Thromb J. 2022;20(1):41.
- Kraaijpoel N, Di Nisio M, Mulder FI, et al. Clinical Impact of Bleeding in Cancer-Associated Venous Thromboembolism: Results from the Hokusai VTE Cancer Study. Thromb Haemost. 2018;118(8):1439-1449.
- Elbadawi A, Shnoda M, Mahmoud K, et al. Efficacy and safety of direct oral anticoagulants vs. low molecular weight heparin for cancer-related venous thromboembolism: a meta-analysis of randomized trials. Eur Heart J Cardiovasc Pharmacother. 202;7(5):380-8.
- Sedaghat M, Lima BS, Bouzari R, et al. Gastrointestinal Bleeding Associated With Warfarin and Rivaroxaban Therapy in Atrial Fibrillation Cases with Concomitant Coagulopathy. Cardiovasc Hematol Disord Drug Targets. 2021;21(2):123-127.
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018;378(7):615-24.
- Tao DL, Olson SR, DeLoughery TG, et al. The efficacy and safety of DOACs versus LMWH for cancer-associated thrombosis: A systematic review and meta-analysis. Eur J Haematol. 2020;105(3):360-362.
- Moik F, Posch F, Zielinski C, et al. Direct oral anticoagulants compared to low-molecular-weight heparin for the treatment of cancer-associated thrombosis: Updated systematic review and meta-analysis of randomized controlled trials. Res Pract Thromb Haemo. 2020;4(4):550-561.
- Hussain MR, Ali FS, Verghese D, et al. Factor Xa inhibitors versus low molecular weight heparin for the treatment of cancer associated venous thromboembolism; A meta-analysis of randomized controlled trials and non-randomized studies. Crit Rev Oncol Hemat. 2020; 169:103526.
