Full HTML
Chronic sinusitis: Decoding the triad of epithelial dysfunction, mucosal inflammation, and microbial dynamics
Sanjay Kumar (Wg Cdr)1 , Kashiroygoud Biradar2
Author Affiliation
1 Associate Professor, Department of ENT,
2 Junior Resident, Department of ENT-HNS, Command Hospital Airforce, Rajiv Gandhi University of Health Sciences, Bengaluru, Karnataka, India
Abstract
Chronic sinusitis, a persistent and long-lasting condition, has a significant effect on an individual’s quality of life. This review offers an in-depth analysis of the complex relationship between epithelial failure, mucosal inflammation, and microbial dynamics, explaining their combined role in the pathophysiology of chronic sinusitis. We address the important role of the epithelial barrier and the effects of its disturbance on immune responses. This review focuses on the mucosal inflammation found in chronic sinusitis cases, highlighting the cytokines and cells that contribute to the ongoing inflammatory response. We provide an in-depth overview of the sinus microbiome’s role in both health and disease states, looking at the effects of bacterial, viral, and fungal colonization on sinus inflammation and their relationship with the epithelial barrier. We also discuss the role of mucociliary clearance and the consequences of its disruption on ongoing inflammation. Each of these factors has a relationship with the others, and together they contribute to chronic inflammation. Current treatment methods are somewhat effective but have limitations. These treatments include methods to improve epithelial function, reduce inflammation, use antibiotics and antifungals, introduce probiotics, and improve mucociliary function. As we learn more, we look at potential new treatments and the need for a complete approach that covers all areas. In short, understanding these areas is key to improving patient care and encouraging more research.
DOI: 10.32677/yjm.v2i3.4296
Keywords: Chronic sinusitis, Epithelial dysfunction, Mucociliary dysfunction, Mucosal inflammation, Sinus microbiome, Therapeutic strategies
Pages: 137-144
View: 3
Download: 5
DOI URL: https://doi.org/10.32677/yjm.v2i3.4296
Publish Date: 19-12-2023
Full Text
Chronic rhinosinusitis (CRS) is a widespread condition characterized by persistent inflammation of the sinuses, affecting approximately 10–12% of the global population [1]. Instead of just being an extended form of acute sinusitis, this condition involves a series of complex pathological changes in the sinonasal region. Clinically, CRS is alarming due to its lasting and often troubling symptoms such as nasal blockage, reduced sense of smell, facial pain, and consistent thick nasal secretion. While these symptoms might seem trivial, they drastically reduce a person’s life quality, matching the decline experienced by individuals with chronic conditions such as congestive heart failure or chronic obstructive pulmonary disease [2]. A closer look at the pathology of CRS uncovers various factors behind its chronic nature. Identifying and grasping these elements is paramount. Such foundational insights guide the formation of treatment strategies. For example, simple inflammation might be tackled with anti-inflammatory medications, but the complex relationship between epithelial malfunction, microbial presence, and mucociliary malfunction necessitates a holistic treatment approach [3]. This review seeks to give a thorough overview of the dynamics underlying CRS. We intend to dissect the contributions of epithelial damage, inherent mucosal inflammation, specific microbial presence, and mucociliary malfunction to chronic sinus inflammation, drawing from the latest research findings and clinical observations. This review aims to deepen understanding, spotlighting consensus, differences, and potential avenues for future research and treatment modalities [4].
SEARCH STRATEGY
An exhaustive literature search was executed across PubMed, Medline, and Scopus databases to probe the complexities of chronic sinusitis. Emphasis was on epithelial malfunction, mucosal inflammation, and microbial interactions. Search terms encompassed “chronic sinusitis,” “epithelial malfunction,” “mucosal inflammation,” “microbial presence,” “mucociliary activity,” and “sinus microbial composition.” Initially, search outcomes were sieved to encompass only articles from the past 10 years to guarantee topicality. Subsequently, abstracts were closely examined for pertinence to the delineated subjects. Non-English language articles and those that deviated from the core themes of the review were excluded. After this rigorous assessment, 34 articles were deemed eligible and included in this literature review. From a detailed review of these 35 articles addressing chronic sinusitis and its association with epithelial dysfunction, mucosal inflammation, and microbial dynamics, we gleaned the following primary insights:
Epithelial Dysfunction in Chronic Sinusitis
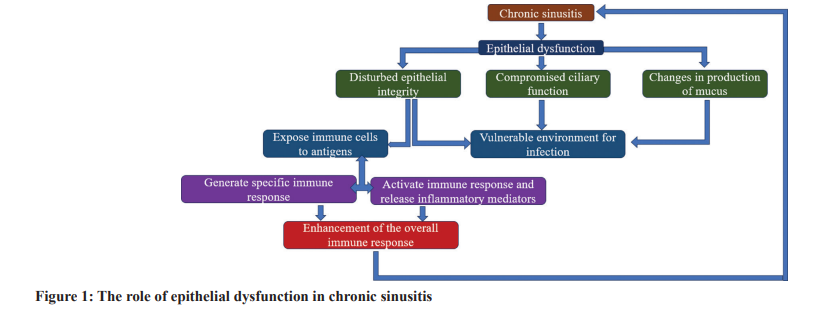
The sinus cavities are encased by epithelial cells, which not only form a protective boundary against external factors but also are vital in sustaining bodily homeostasis. Under standard conditions, this epithelial layer stands at the forefront against potential pathogens and environmental contaminants, simultaneously aiding essential physiological functions, such as mucus production and ciliary motion. When the functions of this layer are diminished, the phenomenon is termed epithelial dysfunction [5]. Epithelial dysfunction, when associated with chronic sinusitis, is typically marked by compromised integrity of the epithelial shield, variations in mucus production, and reduced ciliary activity. Such dysfunctions make the sinonasal region increasingly vulnerable to infections, allergenic reactions, and other irritants. A weakened epithelial defense can facilitate the entry of pathogens, thereby triggering inflammation. Furthermore, sustained sinus inflammation might further harm epithelial structures, creating a reinforcing cycle of consistent inflammation and degradation of the epithelial tissue [6]. The epithelium’s significance transcends its mere physical barrier role; it is crucial for guiding immunological responses. A disrupted epithelial boundary can expose underlying immune cells to foreign antigens, potentially activating diverse immunological cascades. This can result in the liberation of inflammatory mediators such as cytokines and chemokines, which can escalate the inflammation and recruit additional immune cells to the impacted tissue, intensifying the inflammatory response. In addition, the epithelium is capable of generating specific immune mediators on detection of threats or damages, thus strengthening the holistic immune response [7]. Thus, grasping the essence of epithelial dysfunction, especially within the sphere of chronic sinusitis, is paramount. The preservation and efficiency of the epithelial barrier stand fundamental for sinonasal well-being and wield significant influence on the initiation, evolution, and intensity of chronic sinus inflammation. Directing further studies and therapeutic strategies toward bolstering epithelial restoration and endurance might pave the way for innovative and effective avenues to address and potentially reverse chronic sinusitis [8]. Fig. 1 summarizes the role of Epithelial Dysfunction in Chronic Sinusitis.
Intrinsic Mucosal Inflammation
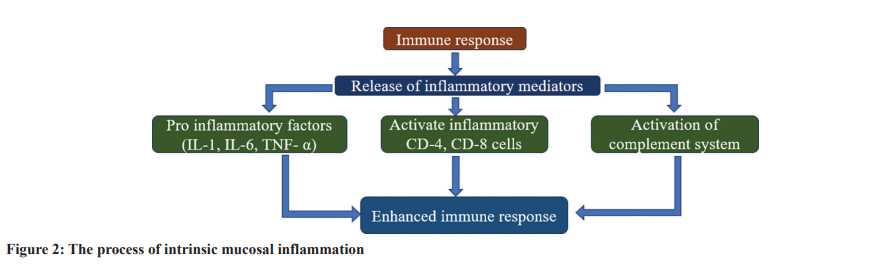
The term “intrinsic mucosal inflammation” delineates the activation of innate immune reactions within the mucosal layer lining several bodily cavities, sinuses included in the study. This type of inflammation is characterized by symptoms such as swelling, erythema, and occasional discomfort in the mucosal regions. Intriguingly, its emergence might not always be attributed to overt external precipitants, such as infections. It somewhat mirrors the body’s immunological counteractions, akin to those observed in autoimmune disorders [9]. The triggering of inherent mucosal inflammation encompasses multiple mechanisms. Central to this are cytokines, these minute proteins, pivotal for intercellular communication. Notably, proinflammatory cytokines, encompassing the likes of interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha, hold the capability to set off and augment the inflammatory cascade. An array of cells, ranging from macrophages, and mast cells to even the epithelial cells, have the potential to secrete these cytokines on encountering specific triggers [10]. Beyond cytokines, myriad elements, comprising immune cells – T- and B-cells alike, alongside segments of the complement system, also play a role in mucosal inflammation. Collectively, these elements orchestrate a state conducive to tissue damage and
diminish the robustness of the mucosal defense, rendering it more prone to external pathogens and allergenic substances [11]. The key challenge with intrinsic mucosal inflammation, especially concerning chronic sinusitis, is its self-sustaining nature. Once the inflammatory response begins, it can potentially weaken the epithelial barrier, permitting allergens, and pathogens to penetrate the underlying tissues. After this primary disruption, a cascade effect can arise, amplifying inflammation, and establishing a cycle where inflammation perpetuates itself. Continuous inflammation might impede the natural repair processes of the epithelium, extending symptoms, and setting the foundation for an enduring problem [12]. Essentially, intrinsic mucosal inflammation holds a central role in the onset and progression of chronic sinusitis. An in-depth grasp of its complexities is crucial, as it can steer the creation of specific therapies aimed at halting the detrimental cycle of inflammation and tissue harm [13]. Fig. 2 explains the intrinsic mucosal inflammation process.
Local Microbial Colonization
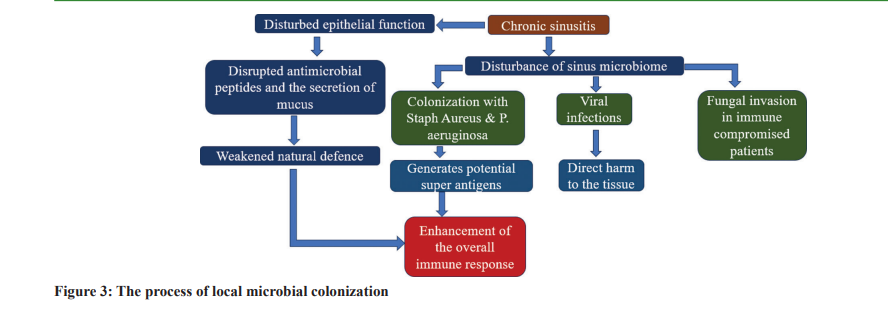
The sinus cavities are not sterile realms. They accommodate a varied assortment of microorganisms, commonly termed the sinus microbiome. When healthy, the microbiome coexists harmoniously with the host, playing defensive roles like countering harmful pathogens and modulating local immune reactions [14]. Nonetheless, any shift in this fine balance can trigger or aggravate sinusitis. The sinus microbiome encompasses bacteria, viruses, and fungi. Its makeup can shift due to factors such as age, environmental interactions, and previous antibiotic treatments. In general, friendly bacteria prevail, aiding in preserving sinus well-being. However, an imbalance, marked by the proliferation of detrimental microbes or a decline in beneficial ones, can render individuals susceptible to infections and inflammation [15]. Bacterial colonization, particularly by aggressive strains such as Staphylococcus aureus or Pseudomonas aeruginosa, can generate toxins and superantigens, eliciting robust immune responses and inflammation. In the same vein, viral intrusions can inflict direct tissue harm and instigate inflammatory routes. Fungi, although rarely associated with healthy people, can still contribute to inflammatory states like fungal sinusitis,primarily in those with weak immune defenses or particular predispositions [16]. The nexus between these microbes and the epithelial defense is intricate. The epithelial stratum stands as the primary shield against microbial adversaries, equipped with inherent immune capabilities to identify and counter threats. This encompasses generating antimicrobial substances and exuding mucus to ensnare and eject pathogens. However, if an anomaly occurs in the epithelial cells, microbes could infiltrate deeper tissue layers, exacerbating or inducing inflammation [17]. In addition, certain pathogens have devised methods to cling to, invade, or dismantle epithelial barriers, adding layers of complexity. In the broader context of chronic sinus inflammation, it is crucial to grasp the intricacies of the sinus microbiome. The inception and evolution of diseases often pivot not only on the presence of pathogens but also on the upheaval of the typically benign microbial assembly [18]. Tackling these microbial skews, either through antibiotic therapies or probiotic applications, might offer innovative avenues for the remedy and deterrence of such disorders. Fig. 3 describes the local microbial colonization process.
Mucociliary Dysfunction
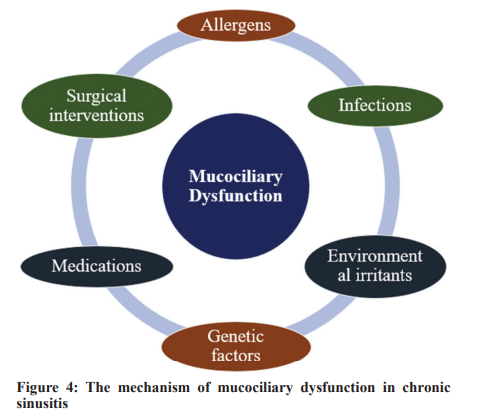
The mucociliary evacuation mechanism holds supreme relevance in safeguarding sinus health. The upper respiratory corridor leans on an efficient protective system consisting of a mucosal film supported by a synchronized set of cilia on the epithelial cells. This complex machinery stands as the frontline shield. The ceaseless motility of mucus, ensnaring particles, microbes, and detritus, progressing toward the pharynx for removal, functions as a vigilant protective barricade against infectious agents and assists in the expulsion of potential nuisances [19]. Nonetheless, this protective efficacy can be critically undermined by mucociliary anomalies. Stasis of mucus in the sinus pockets arises when cilia display flawed or null motion, or when mucus exhibits aberrant fluidity, turning exceedingly viscous or watery. The emergence of inert mucus crafts a milieu apt for the bloom of bacteria, viruses, and fungi, thereby augmenting the risk of recurring infections. In addition, the burden of amassed mucus and its accompanying microbes can incite extended inflammation, culminating in added injury to
the sinus structures, and amplifying the derangement of the mucociliary system [20].
The following etiological factors, among others, have the potential to disrupt the mucociliary machinery:
• Infections: Cilia activity can be undermined by both viral and bacterial infections, either through direct obliteration or indirectly through the spread of toxins and inflammatory agents [21]
• Allergens: Encounter with allergens can cause inflammatory reactions, leading to edematous changes in the sinus epithelium and profuse mucus secretion. Such changes can hinder the normal function of cilia [22]
• Environmental irritants: Toxins, cigarette fumes, and other compounds can injure the cilia and skew the regular texture of mucus [23]
• Genetic elements: They are instrumental in the genesis of specific ailments, like cystic fibrosis. These disorders manifest due to genetic irregularities, leading to the generation of unusually viscous mucus. As a result, the cilia struggle to adeptly mobilize this altered mucus [24]
• Pharmaceuticals: Some drugs, such as antihistamines or those flaunting anticholinergic characteristics, can attenuate mucus production or adjust its composition, thus hindering its movement [25]
• Surgical maneuvers: Procedures, like sinus surgery, can potentially affect mucociliary activity temporarily, due to immediate harm or inflammation post-surgery [26].
Understanding the dysfunction of the mucociliary clearance process and the various elements that contribute to it is paramount given its critical role in maintaining sinus health. The treatment and potential reversal of chronic sinusitis can be significantly impacted by treatment strategies that specifically target and correct mucociliary dysfunction. Fig. 4 describes the factors potentially affecting the mucociliary apparatus.
The Interplay Between These Factors
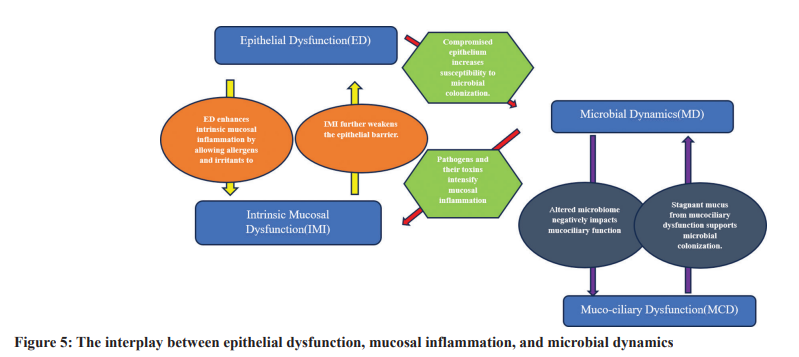
The intricate relationship between epithelial dysfunction, intrinsic mucosal inflammation, local microbial colonization, and mucociliary dysfunction significantly influences the pathogenesis
of chronic sinus inflammation. These elements do not operate in isolation but synergize, often leading to a vicious cycle that culminates in sustained inflammation and long-term health issues. Impaired epithelial function can weaken the primary defensive mechanism of the sinus lining, making it more susceptible to microbial colonization. This weakened barrier allows increased infiltration of allergens, viruses, and irritants, leading to intrinsic mucosal inflammation [5]. This inflammatory response further exacerbates the compromised state of the epithelial barrier, perpetuating a cycle of damage and vulnerability. The sinus’s microbial colonization is a natural biological process. However, when the protective epithelial barrier is impaired, there is a heightened risk of pathogenic overgrowth. Such pathogens can produce toxins and inflammatory mediators, which can amplify the existing inflammation of the mucosal lining [16]. Moreover, a disrupted microbiome might negatively affect mucociliary function, leading to inefficient mucus and microbial removal, making the sinuses prone to recurring infections [14]. The dysfunction of the mucociliary system is a pivotal factor in this chain of events. Even when the epithelial barrier is somewhat
intact or microbial colonization is minimal, a dysfunctional mucociliary system can cause mucus stagnation. This stagnant mucus offers an ideal environment for pathogen growth and persistent inflammation [23]. Chronic sinus inflammation is the result of the cumulative impact of these variables. The chronic nature of the disease arises from the self-sustaining interactions that unfold. As one component intensifies, it enhances the impacts of the others, creating an environment conducive to the continuation of inflammation and the manifestation of symptomatic disease. Considering the intertwined nature of these factors, managing chronic sinus inflammation demands a comprehensive, allencompassing approach. Addressing only one facet, such as microbial colonization, while neglecting the vital roles of epithelial integrity and mucociliary function, might yield only short-term relief. For the formulation of holistic and effective treatment strategies, a profound grasp of the entire problem is imperative. Hence, medical practitioners and researchers need to perceive chronic sinus inflammation not as an isolated ailment but as a hub of interconnected pathological processes, where each element reinforces the others. Embracing this encompassing perspective is essential for devising more effective treatments and enhancing patient outcomes [1]. Fig. 5 describes the interplay between epithelial dysfunction, mucosal inflammation, and microbial dynamics.
Current Therapeutic Approaches and their Limitations
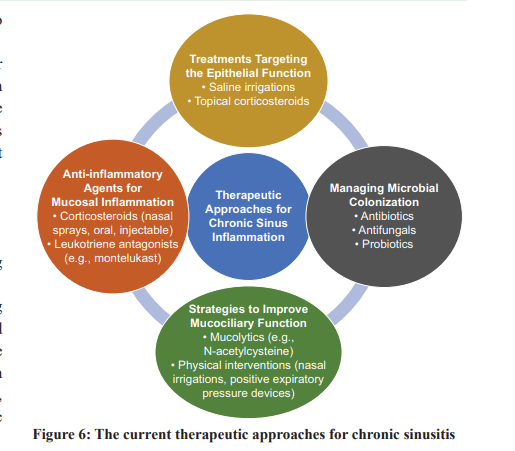
Due to its composite nature, several therapeutic techniques have been employed in the management of chronic sinus inflammation. The treatment options presented in this study aim to address various aspects of disease progression, including epithelial dysfunction, mucosal inflammation, microbial colonization, and mucociliary dysfunction. Various approaches and their inherent limitations are as follows:
1. Treatments Targeting the Epithelial Function: A specific area of focus in medical treatments is interventions that target the activity of epithelial cells. Saline irrigations, especiallyhypertonic saline solutions, are commonly recommended to hydrate the mucosa and enhance epithelial functionality. While topical corticosteroids can mitigate inflammation and improve the integrity of the epithelial barrier [27], they may not always fully restore the normal functioning of the epithelial cells. Prolonged usage might also lead to adverse reactions or diminished effectiveness.
2. The Role of Anti-inflammatory Agents in Modulating Mucosal Inflammation: Corticosteroids, whether administered topically as nasal sprays or systemically through oral or injectable routes, remain the primary approach to combat mucosal inflammation. Leukotriene antagonists, such as montelukast, have shown benefits in individuals with concurrent asthma or aspirin-exacerbated respiratory disease [28]. While corticosteroids are undeniably beneficial, their long-term use can result in systemic side effects, including weight gain, hypertension, osteoporosis, and immunosuppression. Moreover, these interventions often address only the symptoms rather than the root cause of the inflammatory response.
3. Antibiotics, Antifungals, and Probiotics in Managing Microbial Colonization: Microbial cultures guide the appropriate treatment for bacterial sinus infections, often leading to antibiotic prescriptions. In contrast, antifungals are used for fungal sinusitis. However, indiscriminate or prolonged use of antibiotics may lead to antibiotic resistance, disruption of the natural microbiota, and heightened vulnerability to fungal infections [29]. While probiotics have proven effective for gut health, their efficacy in sinus health is still under investigation. Some evidence suggests their potential to restore a balanced sinus microbiome, but further research is essential [30].
4. Strategies to Improve Mucociliary Function: Mucolytics, like N-acetylcysteine, help reduce mucus viscosity and ease its elimination [31]. Physical interventions, including nasal irrigations and positive expiratory pressure devices, can further support mucociliary clearance. Nonetheless, these interventions might not consistently return mucociliary
function to its optimal state, necessitating regular use to maintain benefits. In summary, while various therapeutic modalities exist for managing chronic sinus inflammation, each comes with its own set of challenges. There’s a clear need for treatments capable of effectively addressing the myriad of underlying causes contributing to this condition. Fig. 6 summarizes the current therapeutic approaches for chronic sinusitis.
Future Directions in Chronic Sinusitis Treatment
1. Potential Novel Treatments or Interventions Targeting Specific Pathways:
• Biologics: The effectiveness of biologics in treating various inflammatory conditions has generated significant interest in their potential therapeutic use for chronic sinusitis. Monoclonal antibodies, which selectively target inflammatory mediators such as IL-4, IL-5, and IL-13, might provide targeted therapeutic interventions to manage mucosal inflammation [32].
• Small Molecule Inhibitors: Janus Kinase Inhibitors are low molecular weight molecules that can selectively target and regulate proteins or enzymes with heightened activity in the inflammatory response linked to sinusitis. By inhibiting pathways responsible for inflammation, these inhibitors can alleviate symptoms, reduce mucosal tissue inflammation, and restore the regular function of the sinus epithelium [33].
• Barrier Repair Therapies: Given the growing understanding of the importance of epithelial dysfunction, there is increased interest in pharmaceutical solutions that boost barrier repair and fortify epithelial function [7].
2. Importance of Holistic Approaches:
• Personalized Medicine: Given the diverse manifestations of chronic sinusitis, it is plausible that future treatment methods might be tailored to individual patient’s genetic makeup, unique inflammatory markers, and patterns of microbial colonization [34].
• Integrative Approaches: Marrying medical therapy with lifestyle interventions – such as dietary changes, physical activity, and environmental modifications – may enhance the effectiveness of treatment outcomes. Fig. 7 shows the future Directions in Chronic Sinusitis Treatment.
ONGOING RESEARCH AND CLINICAL TRIALS
• Microbiome Manipulation: The study of probiotics has garnered significant interest in evaluating the potential benefits of precise manipulation of the sinus microbiome for individuals with chronic sinusitis. Such changes might be achieved through the application of microbial transplants, akin to the methods used in fecal transplants [14].
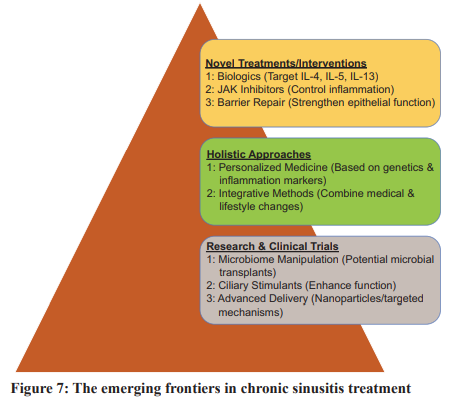
• Ciliary Stimulants: Considering the critical role of the mucociliary apparatus, medications designed to stimulate or enhance ciliary function are under exploration [25].
• Advanced Delivery Systems: To improve drug delivery to sinus tissues, research is focusing on advanced systems, such as nanoparticles or targeted delivery mechanisms, ensuring that drugs are delivered to their intended location in optimal concentrations [34]. In conclusion, the future of chronic sinusitis treatment holds promise. The fusion of technological advancements, genetic research, and a deeper understanding of disease pathophysiology places us on the cusp of a transformative era in healthcare. This era promises treatments that are more precise, effective, and holistic. As science delves deeper into the intricacies of chronic sinusitis, those affected by this condition can look forward to more refined and effective therapeutic options in the near future.
LIMITATIONS AND FALLACIES
Our review, which is predominantly from PubMed and Medline, may have selection bias due to the omission of studies from other databases. By limiting our review to English-language articles, we may have missed relevant non-English studies. The differences in study design and methodology of the selected articles may make it difficult to draw consistent conclusions. Our review represents data up to a point in time and may ignore current developments. In addition, there may be a disproportionate focus on newer, less validated treatments, which could distort our perception of their true efficiency
CONCLUSION
Chronic sinusitis is not simply an ailment of the sinuses; it is a culmination of intricate interactions that involve epithelial malfunction, intrinsic mucosal inflammation, microbial colonization, and mucociliary dysfunction. Grasping the nuanced interplay between these factors is vital for effective patient management and elevating the quality of life for the afflicted. The wide array of therapeutic approaches available – ranging from drugs targeting epithelial function and inflammation to interventions addressing microbial colonization and mucociliary processes – reflects the multifaceted nature of the disease. While these methods have brought relief to many, they also underscore the gaps in our current understanding and the limitations of our therapeutic arsenal. Our understanding of the subject is still evolving. The complex and diverse nature of chronic sinusitis requires a holistic research approach. The deeper we delve into the cellular, molecular, and microbiological aspects of this disease, the more promising it is to develop treatments that are both more targeted and more effective
AUTHORS’ CONTRIBUTIONS
All authors contributed to the completion of this work. The final manuscript was read and approved by all authors
References
1. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020;58:1-464.
2. Rudmik L, Smith TL. Quality of life in patients with chronic rhinosinusitis. Current Allergy Asthma Rep 2011;11:247-52.
3. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy Clin Immunol 2015;136:1431-40.
4. Cho SH, Hong SJ, Han B, et al. Age-related differences in the pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol 2012;129:858-60.
5. Steelant B, Seys SF, Boeckxstaens G, et al. Restoring airway epithelial barrier dysfunction: A new therapeutic challenge in allergic airway disease. Rhinology 2016;54:195-205.
6. Wira CR, Fahey JV, Rodriguez-Garcia M, et al. Regulation of mucosal immunity in the female reproductive tract: The role of sex hormones in immune protection against sexually transmitted pathogens. Am J Reprod Immunol 2014;72:236-58.
7. Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol 2012;130:1087-96.e10.
8. Schleimer RP, Kato A, Kern R, et al. Epithelium: At the interface of innate and adaptive immune responses. J Allergy Clin Immunol 2007;120:1279-84.
9. Woof JM, Mestecky J. Mucosal immunoglobulins. In: Mucosal Immunol. Boston: Academic Press; 2015. p. 287-324. 10. Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428-35.
11. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783-801.
12. Bachert C, Zhang N, Patou J, et al. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol 2008;8:34-8.
13. Xiao W, Hodge DR, Wang L, et al. NF-kappaB activates IL-6 expression through cooperation with c-Jun and IL6-AP1 site, but is independent of its IL6-NFkappaB regulatory site in autocrine human multiple myeloma cells. Cancer Biol Ther 2004;3:1007-17.
14. Abreu NA, Nagalingam NA, Song Y, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med 2012;4:151ra124.
15. Ramakrishnan VR, Hauser LJ, Feazel LM, et al. Sinus microbiota varies among chronic rhinosinusitis phenotypes and predicts surgical outcome. J Allergy Clin Immunol 2015;136:334-42.e1.
16. Cleland EJ, Bassiouni A, Vreugde S, et al. The bacterial microbiome in chronic rhinosinusitis: Richness, diversity, postoperative changes, and patient outcomes. Am J Rhinol Allergy 2016;30:37-43.
17. Choi EB, Hong SW, Kim DK, et al. Decreased diversity of nasal microbiota and their secreted extracellular vesicles in patients with chronic rhinosinusitis based on a metagenomic analysis. Allergy 2014;69:517-26.
18. Cope EK, Goldberg AN, Pletcher SD, et al. Compositionally and functionally distinct sinus microbiota in chronic rhinosinusitis patients have immunological and clinically divergent consequences. Microbiome 2017;5:53.
19. Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol 2007;69:401-22.
20. Workman AD, Brooks SG, Kohanski MA, et al. Bitter and sweet taste tests are reflective of disease status in chronic rhinosinusitis. J Allergy Clin Immunol Pract 2018;6:1078-80.
21. Sisson JH, Stoner JA, Ammons BA, et al. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 2003;211:103-11.
22. Smith K, Burt R, Nair S. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun 2011;12:466-73.
23. Ramanathan M Jr., Lane AP. Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol Head Neck Surg 2007;136:348-56.
24. Nonaka M, Pawankar R, Tomiyama S, et al. Proliferative effect of estradiol on human nasal epithelial cells in postmenopausal women. Auris Nasus Larynx 2010;37:576-81.
25. Chotirmall SH, O’Donoghue E, Bennett K, et al. Sputum Candida albicans presages FEV₁ decline and hospital-treated exacerbations in cystic fibrosis. Chest 2010;138:1186-95.
26. Henriquez OA, Den Beste K, Hoddeson EK, et al. House dust mite allergen Der p 1 effects on sinonasal epithelial tight junctions. Int Forum Allergy Rhinol 2013;3:630-5.
27. Mayer AK, Muehmer M, Mages J, et al. Differential recognition of TLRdependent microbial ligands in human bronchial epithelial cells. J Immunol 2007;178:3134-42.
28. Ramanathan M Jr., Spannhake EW, Lane AP. Chronic rhinosinusitis with nasal polyps is associated with decreased expression of mucosal interleukin 22 receptor. Laryngoscope 2007;117:1839-43.
29. Ramanathan M Jr., Lee WK, Dubin MG, et al. Sinonasal epithelial cell expression of toll-like receptor 9 is decreased in chronic rhinosinusitis with polyps. Am J Rhinol 2007;21:110-6.
30. Pezato R, Swierczynska-Krepa M, Niemczyk K, et al. Why has nature evolved such a gigantic air conditioning system as the human nose? Rhinology 2014;52:3-7.
31. Psaltis AJ, Weitzel EK, Ha KR, et al. The effect of bacterial biofilms on post-sinus surgical outcomes. Am J Rhinol 2008;22:1-6.
32. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper onrhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012;50:1-12.
33. London NR Jr., Ramanathan M Jr. The role of the sinonasal epithelium in allergic rhinitis. Otolaryngol Clin North Am 2007;40:549-63.
34. Song WJ, Sintobin I, Sohn KH, et al. Staphylococcal enterotoxin IgE sensitization in late-onset severe eosinophilic asthma in the elderly. Clin Exp Allergy 2016;46:411-21.
