Full HTML
Abruptio placenta and pregnancy outcome at Al-Sadka teaching hospital, Aden, Yemen
Rehab Ahmed Omer Bamhdi1, Fatima Gamal-Aleil1
Author Affiliation
1Consultant, Department of Obstetrics and Gynecology, Al-Sadka Teaching Hospital, Aden, Yemen
Abstract
Background: Placental abruption is a serious obstetric complication with many potential adverse outcomes for both mother and fetus. The aim of this study was to determine the clinical characteristics, risk factors and maternal and perinatal consequences of placental abruption among pregnant women, at Al-Sadka Teaching Hospital. Methods: This was a prospective descriptive study including women with placental abruption, who delivered at Al-Sadka University Hospital, Aden, Yemen, during the period from August 1, 2015, to September 31, 2016. Statistical analysis of the data was performed with (SPSS version 24). Results: The frequency of placental abruption in this study was 0.84%. The majority of women with placental abruption were in the 20-25 age group (34.3%), multiparous (51.4%), had no antenatal care (51.4%). and illiterate (37.1%). The most common clinical presentation was vaginal bleeding (82.9%), followed by tetanic uterine contractions (68.6%). The most common risk factors associated with placental abruption were preeclampsia (34.5%) and PROM (21.8%). Most of our patients (77.1%) had a vaginal delivery, while the rest had a cesarean section delivery. The most common maternal complications were postpartum hemorrhage (100%) and hemorrhagic shock (27.5%). Perinatal death was more commonly associated with preeclampsia (55.0%), severe placental abruption (95%), and preterm birth (70%). On the other hand, perinatal death was reported in 28.6% of our patients (n=20). A higher percentage of fetuses born by caesarean section died (56.3%), while 79.6% of vaginal deliveries involved live fetuses. Conclusion: The frequency of placental abruption was 0.84%. Women with AP had a higher likelihood of being illiterate, between 20 and 25 years old, multipara, and receiving inadequate antenatal care. Accordingly, we recommend raising women's awareness of the importance of antenatal care through the media (television and radio) and creating policies to encourage high-quality routine prenatal care for all pregnant women by policymakers, particularly the Ministry of Public Health.
DOI: 10.32677/yjm.v2i2.4196
Keywords: Abruptio placenta, High risk, Maternal mortality, Pregnancy outcomes
Pages: 87-94
View: 4
Download: 6
DOI URL: https://doi.org/10.32677/yjm.v2i2.4196
Publish Date: 28-09-2023
Full Text
INTRODUCTION
Abruptio placenta (AP) also known as placental abruption, is the premature separation of the normally implanted placenta from the uterine wall [1]. It is potentially disastrous to both the fetus and the mother with significant morbidities and mortalities. Maternal risks associated with AP include massive blood loss, disseminated intravascular coagulopathy, renal failure, and, less commonly, maternal death [1,2], while fetal risks are associated with intrauterine growth restriction, low birthweight, preterm delivery, asphyxia, stillbirth and perinatal death [1,2].
The incidence of AP varies slightly in different populations, it occurs in 0.4–1% of pregnancies [2]. There are several risk factors that have been linked to AP as maternal smoking, hypertensive disorders of pregnancy (mostly superimposed preeclampsia), premature preterm rupture of membranes (PPROM), history of previous AP, current diabetes mellitus, previous history of cesarean section, trauma and thrombophilic disorders [1-5].
There are few published studies on AP in Yemen. The aim of this study was to determine the clinical features, risk factors, and maternal and perinatal consequences of AP in pregnant women between August 2015 and September 2016 at Al-Sadaka Teaching Hospital, Aden, Yemen.
PATIENTS AND MATERIALS
Study design, population, and setting:
This descriptive, prospective, hospital-based study was conducted at Al-Sadka Teaching Hospital between August 1, 2015, and September 31, 2016, at Al-Sadka Teaching Hospital, Aden, Yemen. All pregnant women who were diagnosed with abruptio placenta (AP) were included in this study. Al-Sadka Teaching Hospital is the main referral hospital for maternal and child care in Aden governorate, Yemen.
Definition of cases
The diagnosis of AP was made on clinical signs and symptoms of vaginal bleeding, tense and tender abdomen, hypertonus uterine contractions, fetal distress, or fetal death and confirmed by the presence of retro-placental clots, detected either ultrasonographically (during pregnancy) or macroscopically (after delivery) [1-3].
Inclusion criteria
1-. Gestational age ≥ 24 weeks.
2-. All Singletons & multiple pregnancy diagnoses with Abruptio placenta.
Exclusion criteria
1- Gestational age less than 24 weeks.
2- Unknown maternal age.
3- Inaccurate gestational age.
4- All other causes of antepartum hemorrhage.
Sample size: A complete enumeration of eligible patients was followed during the study period.
Definition of main terminology and study variables used
Age: Complete years from her birth until the time of delivery. If do not remember the date of birth, it will be approximated by asking the age of the first child. In this study, maternal age was grouped as follows: [6]
< 20
20 – 25
26 – 30
31 – 35
>35
Gestational age: Gestational age is the time measured from the first day of the woman's last menstrual period (LMP) to the current date. It is measured in weeks, if the LMP is unknown, gestational age was calculated by early ultrasound [7,8].
Parity: It is defined as the number of times that she has given birth to a fetus or fetus that reached viability with a gestational age of 24 weeks or more, regardless of whether the child was born alive or was a stillborn infant (weighing 500 gm or more). It was classified as: [9]
· Nullipara: 0 birth.
· Multipara: 1-4 births.
· Grand multipara: ≥5 births.
Antenatal care: Systemic monitoring (examination and counseling) of women during pregnancy, including education, counseling, screening, and treatment to ensure a normal pregnancy with the birth of a healthy baby from a healthy mother. It should be regular and periodic and based on the needs of the individual. Indeed, prenatal care is care on a continuum, beginning before pregnancy and ending with delivery and the postpartum period [10]. The quality of antenatal visits was described as poor when there were three or fewer visits.
Maternal education: Refer to the highest education levels for which respondents have obtained. In our study, we included four categories: illiterates, primary school (including reading and writing), secondary school (including preparatory), and university.
Preeclampsia: Preeclampsia is a disorder of pregnancy that is associated with new-onset hypertension (defined as a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg on two occasions at least 4 hours apart), most often after 20 weeks gestation and frequently near term, accompanied by new-onset proteinuria, with greater than or equal to 300mg urine protein excretion in 24 hours or a protein/creatinine ratio of greater than or equal to 0.3. A urine dipstick can be used if the other methods are not available and proteinuria is defined as a protein reading of at least 1+ [11].
Gestational hypertension: Elevation of blood pressure during the second half of pregnancy or in the first 24 hours postpartum without proteinuria and without other signs and symptoms of preeclampsia or preexisting hypertension. In this study, the diagnosis was based on a documented increase in blood pressure for the first time during pregnancy either during antenatal care or at the time of admission, without proteinuria [11].
Superimposed preeclampsia: Preeclampsia that occurs in women with pre-existing chronic hypertension [11].
Chronic hypertension: Chronic hypertension is defined as hypertension present before pregnancy, or before 20 weeks of gestation, that persists more than 3 months postpartum [11].
Diabetes mellitus: Diabetes mellitus was diagnosed if there was a history of diabetes mellitus or if the patient was receiving drugs for diabetes or their random blood sugar levels were above 200 mg (11.1 mmol/L) with symptoms, or if fasting blood sugar scored more than 126 mg (7 mmol/L) or Hb A1c > 6.5% for more than two readings during hospitalization [12].
Premature rupture of membrane (PROM): It is defined as the rupture of the membranous sac that surrounds a fetus (amniotic sac) that occurs spontaneously before the onset of labor. This condition was diagnosed clinically from the history of sudden gush of fluid from the vagina. A sterile speculum examination revealed cervix leakage and amniotic fluid pooling in the posterior fornix [13].
Placenta previa: Placenta previa is a condition in which the placenta lies in the lower uterine segment, completely or partially obstructing the internal os of the cervix. [14].
Fetal distress: Fetal distress, also known as “non-reassuring fetal heart tracing”, is an ill-defined term, used to express intrauterine fetal jeopardy, a result of intrauterine fetal hypoxia [15]. In this study, fetal distress was diagnosed clinically by intermittent auscultation of the fetal heart every 30 minutes in the first stage and every 15 minutes in the second stage. Auscultation was performed for 60 seconds during and after each uterine contraction. Fetal bradycardia is defined as a fetal heart rate less than 110 beats per minute, while tachycardia is defined as a fetal heart rate greater than 160 beats per minute..
Perinatal mortality: It is defined as late fetal deaths at 28 completed weeks of gestation or more and early neonatal deaths under age 7 days [16].
Still birth: Delivery of a neonate after 28 weeks with no signs of life either during pregnancy or during labor [17].
Apgar score: This scoring system is a useful clinical tool to identify those neonates who require resuscitation as well as to assess the effectiveness of any resuscitative measures Apgar, 1953). As shown in Table, each of the five easily identifiable characteristics: heart rate, respiratory effort, muscle tone, reflex irritability, and color—is assessed and assigned a value of 0 to 2. The total score, based on the sum of the five components, is determined 1 and 5 minutes after delivery. In this study, we coded 1st and 5th minute Apgar score [18].
Birth weight: It refer to the first weight of fetus or newborn obtained after birth. Preferable to be measured within the first hour of life before significant post natal weight loss has occurred [19].
Maternal mortality: It is defined as maternal deaths occurring during pregnancy or within 42 days of its termination, irrespective of duration or site of pregnancy, from any cause related to or aggravated by the pregnancy or its management but not from accidental or incidental causes [20].
Postpartum hemorrhage any amount of blood from or into the genital tract following birth of the baby up to the end of puerperium, which adversely affects the general condition of the patient, evidence by increase in pulse rate, falling in blood pressure and the need for blood transfusion. The average blood loss following vaginal delivery, cesarean delivery and cesarean hysterectomy is 500 mL, 1000 mL and 1500 mL respectively However, this definition was taken for the purpose of this study [21].
Couvelaire uterus: Also known as uteroplacental apoplexy. It is rare but is seen in severe AP, where blood leaks from the clot into the myometrium. The appearance resembles a blue-tinged, large, swampy uterus. It is a clinical diagnosis made during a visual examination of the uterus. However, with a biopsy, the presence of heme throughout the myometrium is detected [22].
Shock: A loosely defined term used to describe the clinical syndrome that develops when oxygen delivery is inadequate to meet the metabolic requirement of the tissue due to some form of acute circulatory failure. Hypovolemic shock is due to major reduction in blood volume. General feature of shock [23]:
· Hypotension (systolic BP100/min).
· Cold skin.
· Rapid, shallow respiration.
· Drowsiness, confusion, irritability.
· Oliguria (urine output <30 ml/hour)
· Elevated or reduced central venous pressure.
Data collection
Data were collected directly from the patients or their relatives by the lead investigator or other trained residents. A special data collection form was developed for this study, which contains the demographic and clinical characteristics of the patients, the results of their investigations, and the course of the pregnancy.
Data analysis
The statistical analysis of the data was performed with (SPSS Version 24). Categorical variables were described as number (n) or proportions (%). The results were presented in tables to summarize the data and to show descriptive relationships between different variables. Chi-square tests or Fisher's exact test were used to determine the association between perinatal death and risk factors for AP. In addition, chi-square tests or Fisher's exact test were used to describe the association between mode of delivery and fetal outcomes. P value < 0.05% was considered statistically significant.
Ethical consideration
The conduct of the study was given approval by Al-Sadka Hospital's administration. The patients were given a thorough explanation of the goals and significance of the current study in a straightforward manner. Therefore, all participants provided their written, informed consent.
RESULTS
Demographic and obstetric characteristics of the study participants
From August 2015 to September 2016, 8298 women gave birth at our hospital. Out of these, 70 women who had abruption placentae (AP) were enrolled in this study, representing a prevalence of approximately 8.4 per 1000 hospital deliveries during the study period. The peak age groups affected by AP were 20 to 25 years and 26 to 30 years (34.3% and 31.3%, respectively). Most of them were residents of Aden and Lahj governorates (70.0% and 18.6% respectively), and 80.0% of them were housewives. Illiteracy was found in 37.1% of cases. We found 44.3% of patients with AP had no particular social habits. Of the rest, 34.3% were qat chewers and 21.4% were smokers. Table 1 summarizes the distribution of participants by age group.
Table 1: Demographic characteristics of participants with abruption placenta
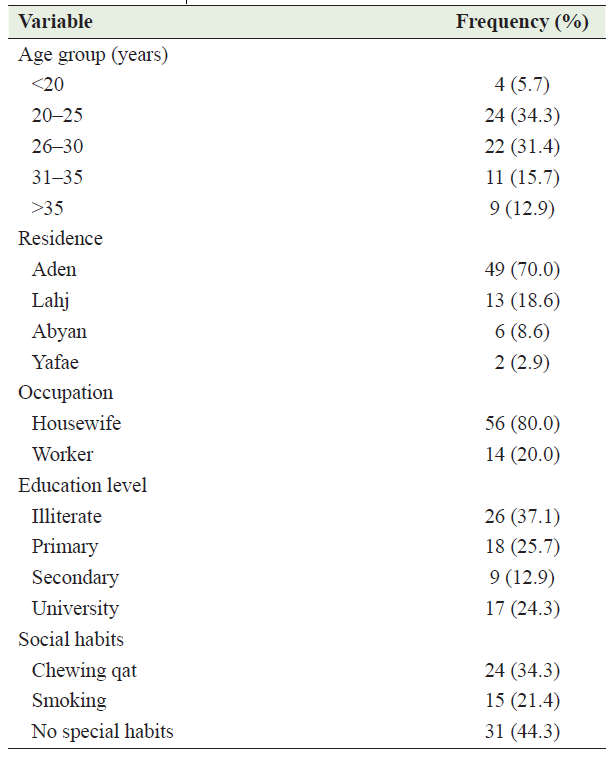
Half of the studied patients with AP (51.4%) did not have antenatal care (ANC) during the current pregnancy, and a quarter (22.9%) did not have ANC adequately. Parity of the studied women was variable, 21.4% were nullipara, 51.4. % were multipara and about 54.3% of them with term pregnancy (37 – 40 weeks). Table 2, describes the obstetric characteristics of the studied participants.
Table 2: Obstetrical characteristics of the participants with abruption placenta
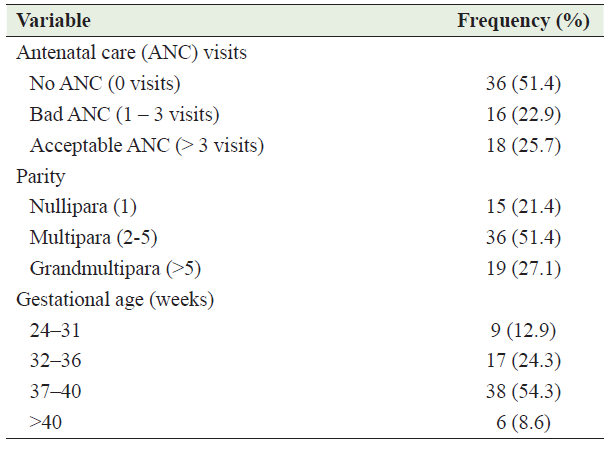
Clinical characteristics of the participants and risk factors for abraptio placenta
The degree of abruption was mild in 24(%), moderate in 22(%), and severe in 24(%) of them. The common clinical presentation was vaginal bleeding which was present in 82.9% of cases followed by a tetanic uterine contraction in 68.6% of them, retro placental hematoma in 57.1%, and Bloodstained amniotic fluid in 41.4% of cases. Other clinical findings included fetal distress in 25.7% and intrauterine fetal death in 21.4% of cases. AP risk factors were identified in 78.6% of patients. The most common risk factors were preeclamptic in 34.5% of cases, premature rupture of membranes (PROM) in 21.8% of cases, previous lower segment cesarean section (LSCS) in 14.5% of cases, and gestational hypertension in 12.7% of patients. Table 3 summarizes the clinical characteristics of the participants and associated risk factors.
Table 3: Clinical characteristics of the participants and risk factors for abraptio placenta
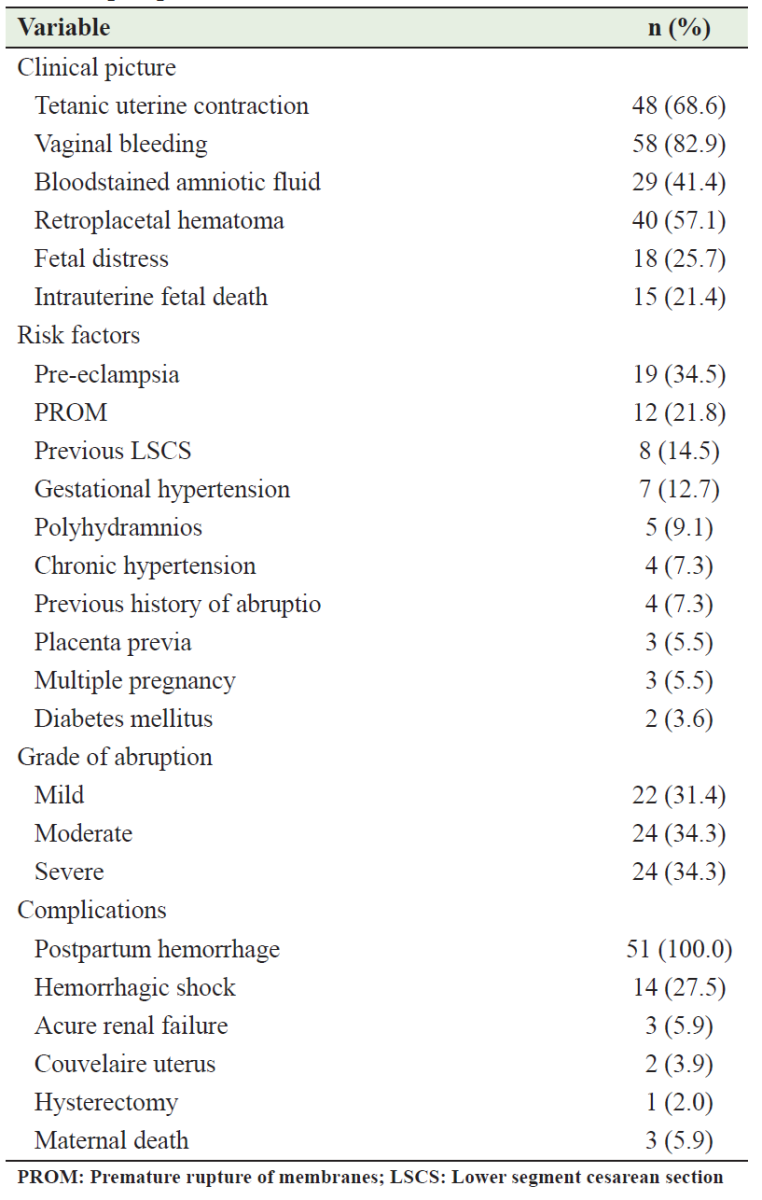
Mode of delivery and complications of abraptio placenta
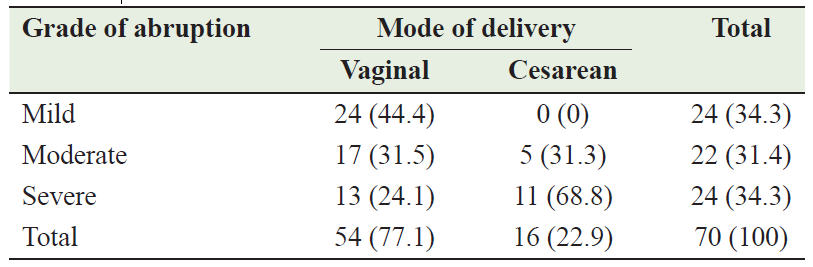
The mode of delivery for most patients with AP was vaginal mode (77.1%; n=54) and the remainder 22.9% (n=16) delivered by Cesarean sections (CS). There is a significant relationship between the mode of delivery and the grade of abruption (p<0.05); all patients with mild abruption delivered vaginally, while most of the patients who delivered by cesarean section 11 (68.8%) had a severe abruption (Table 4). The total CS performed during the study period was 1533 in Al-Sadka Teaching Hospital; the percentage of CS attributed to AP was 1.04% of the total CS performed during the same period.
Table 4: Mode of delivery according to the grade of abruption placenta
Complications were identified in 72.9% of the participants (Table 5). Postpartum hemorrhage was observed in all patients (100.0%), followed by hemorrhagic shock 14 (27.5%). Acute renal failure was reported in 3 (5.9%) patients, and a Couvelaire uterus was found in 2 (3.9%), while a hysterectomy was carried out in one patient (2.0%). Maternal death was reported in 3 (5.9%) cases.
Fetal outcomes
As described in Table 5, it was found that among the 20 perinatal deaths, the risk factors in the studied patients with AP were preeclampsia in 11 (55%) cases, PROM in 5 (25%) patients, previous LSCS, gestational hypertension, and chronic hypertension in 10% of cases for each. Among these risk factors, preeclampsia demonstrated a statistically significant correlation with fetal death in the studied patients (p=0.001). When the gestational age of the patients under study was correlated with fetal outcome, the perinatal deaths of 14 (70%) patients occurred during preterm gestations (24 to 36 weeks), while 30% of cases occurred during full-term gestations (37-40 weeks), which was statistically significant (p=0.002). In patients whose gestation lasted more than 40 weeks, no perinatal deaths were reported (Table 5). In terms of the grade of abruption, it was found that the majority of perinatal deaths occurred in mothers with severe abruption (95.05%), while the percentage of surviving fetuses with severe placental abruption was 10% of all living fetuses. Statistically, there is a significant association between the grade of abortion and fetal outcome (p<0.01) (Table 5).
Table 5: Distribution of AP risk factors in relation to fetal outcomes
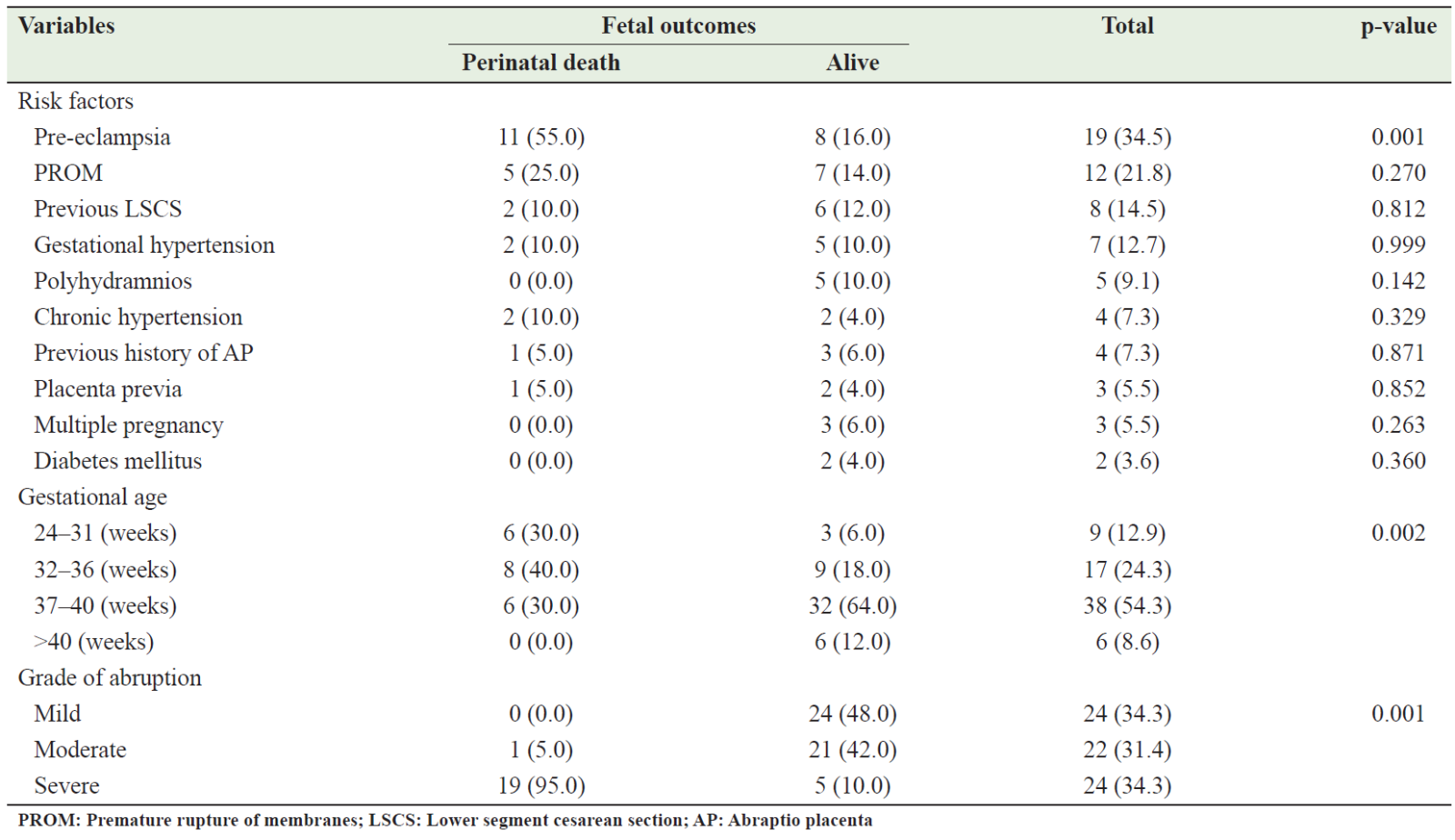
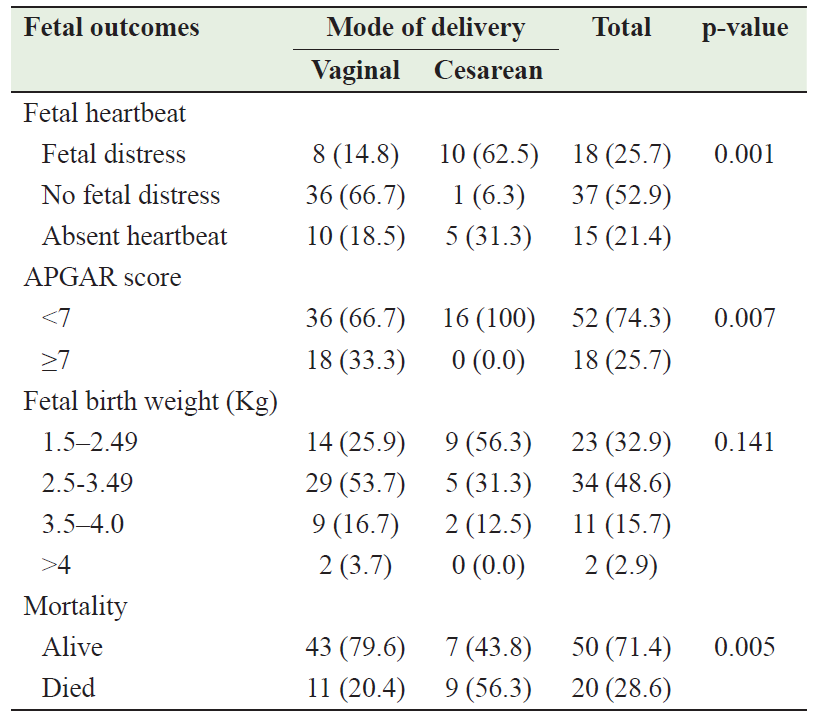
Table 6 describes the relationship between mode of delivery and fetal outcomes. Regarding the heartbeat, 52.9% of the delivered fetuses had a normal heart rate, 25.7% had fetal distress, and 21.4% had no heartbeat. There is significant relationship between the mode of delivery and fetal heart beat (p<0.01). For the APGAR score; there was a significant relationship between mode of delivery and APGAR score (p<0.01); all cesarean deliveries resulted in fetuses with a score <7, while 33.3% of vaginal deliveries resulted in fetuses with a score ≥7. On the other hand, there was no significant relationship between mode of delivery and fetal birth weight (p=0.141). There was a significant association between the mode of delivery and fetal outcome; a higher percentage of fetuses born by cesarean section died compared with vaginal delivery [9 (56.3) vs 11 (20.4); p=0.005].
Table 6: Fetal outcomes in relation to mode of delivery
DISCUSSION
Abruptio placenta (AP) is a serious obstetric complication that remains a leading cause of maternal and perinatal morbidity and mortality worldwide, particularly in developing countries. This study is one of the few studies designed to investigate AP in Yemen. As our medical center is the only tertiary hospital serving the entire population of the southern governorates in Yemen, our data allows us to study the prevalence of AP and its risk factors and assess their impact on maternal and fetal outcomes.
In this study, the prevalence of AP was 0.84%. This result is consistent with the results of other studies from Iran, Pakistan, India, and Norway, which showed AP frequencies of 1%, 1.03%, and 0.4%, respectively [24,25,26]. On the other hand, higher frequencies were reported in other studies from Pakistan, India, and Sudan, which reported AP rates of 3.7% [27], 4.4% [28], and 6.5% [29], respectively. These variations in frequency could be due to differences in diagnostic criteria used or differences in study populations, or insufficient recording of AP cases in some centers.
In the present study, we found that most cases of AP occur in maternal age between 20 and 25 years with a frequency of 34.3%. However, high frequencies of AP were reported in this age group by Saeed et al (50%) [30], Barua et al (66.6%) [31], and Hossain et al (72.2%) [27]. Although advanced maternal age and high parity are well-documented risk factors for AP, our study showed that most cases of placental AP occur in the maternal age group between 20 and 25 years, which can be explained by the fact that early marriage in our population results in high parity in this age group.
One of the most important risk factors for AP is definitely the hypertensive disorders of pregnancy. A recent meta-analysis has shown that hypertensive disorders of pregnancy including gestational hypertension, preeclampsia and eclampsia increase the risk of PA by almost 3 times (OR 2.79, 95% CI 2.37; 3.27) [32]. However, the frequency of hypertensive disorders of pregnancy varies among patients with PA in different studies worldwide. In our study, it was found in 42.8% (n=30) of cases, while it was found in about 80% of cases in other study [33]. Among hypertensive disorders of pregnancy phenotypes, pre-eclampsia was found as the most important risk factor for AP. Nankali et. al., demonstrated a 7.7% risk of AP in severe preeclampsia [34]. In our study pre-eclampsia was found in 34.5% of cases, while in Dubai it was found in 19.5% [35], in India in 67% [36], and in Ghana 29% [37]. Smoking is a known risk factor for AP [38], with the relative risk ranging from 1.4 to 2.5. [39-41] The risk of AP rises with daily cigarette consumption, [41] reaching a plateau at around 10 cigarettes per day after which the risk is constant. [42]. According to some studies, smoking frequency among women with AP ranged from 1.2 to 37.2% [27,33]. Our study found that (21.4%) of the women with PA were smokers, which is within the global range.
As noted, all of these predisposing risk factors are preventable or at least controllable with better antenatal care (ANC). According to the results of this study, 51.4% of the women did not receive adequate ANC. This finding is similar to that of Bibi et al. and Dars et al., who found that 35% and 91.3%, respectively, did not receive adequate ANC. [43,44] In addition, Abbasi et al. and Budde et al. found that the majority of women lacked ANC [45,46]. This result could be explained by the fact that the majority of the women in the current study were illiterate, which is attributed to the situation in our country.
Regarding the mode of delivery, our study showed that the majority of women gave birth vaginally (77.1%). This result is comparable to other studies showing vaginal delivery as the main method of delivery [27, 43, 44], but in contrast to Tikkanen et al. and Saquib S et al, who reported a cesarean section (CS) rate of 91%, and 78% respectively [1,35]. Indication of CS in patients with AP varied from study to study, most likely it is attributed to the viability of the fetus at the time of presentation. Since the time from presentation to the birth has a major impact on fetal well-being, an emergency CS may be the prompt choice for practitioners, who believe that the CS is the best management for AP with a live fetus. Our study showed high perinatal mortalities among women who gave birth through CS (Table 6).
AP is associated with increased maternal complications. The most frequent complication of AP in current study was postpartum hemorrhage with frequency of 100%. The frequency of this complication varied in different studies depending on the patient status at presentation time, the available medical facilities and efficiency of healthcare workers. Other studies reported rate of postpartum hemorrhage in AP cases from 12% to 36.3% [30, 47,48].
For both the mother and the fetus, AP is linked to mortality rates of varying percentages. AP is associated with maternal and fetal mortality, which varies from study to study. According to various studies, maternal mortality varied between 2% and 32% [27, 49-52]. In the current study, the maternal mortality rate was 5.99% (n=3) and is therefore within the above range. In AP, uncontrolled hemorrhagic shock, DIC, postpartum hemorrhage, or acute renal failure are the leading causes of maternal death. On the other hand, AP can affect the fetus causing preterm labour, low birth weight, and fetal death. Perinatal outcomes may vary from study to study. Our study showed that perinatal outcomes depend on risk factors such as preeclampsia, the gestational week at which AP developed, and the grade of abruption (Table 5). In developed countries, perinatal mortality attributed to AP is between 9% and 12%, while in developing countries it can reach up to 60% [51]. In the present study, perinatal mortality was observed in 50 cases (71.4%).
This study has some limitations. First, this study was hospital-based and it did not reflect the community situation, therefore, we cannot generelize our results. Second, the data was relatively old. However, we believe that our data are novel, due to the scarcity of studies in this field, and can be used as a reference for any future study.
CONCLUSION
In our setup, the frequency of AP was 0.84%, which is comparable with local and international literature. Women with AP had a higher likelihood of being illiterate, between 20 and 25 years old, multipara, and receiving inadequate antenatal care. Vaginal bleeding associated with tetanic uterine contraction should alert the obstetrician to AP because the diagnosis is still clinical. The most frequent maternal-associated risk factor was pregnancy-induced hypertension, and vaginal birth was the frequent mode of delivery. Postpartum hemorrhage and hemorrhagic shock were the most common maternal complications. Prematurity and preeclampsia were associated with increased perinatal mortality and there was a significant relationship between the grade of AP and perinatal mortality, with severe AP increasing the number of dead fetuses. Accordingly, we recommend raising women's awareness of the importance of prenatal care through the media (television and radio) and creating policies to encourage high-quality routine prenatal care for all pregnant women by policymakers, particularly the Ministry of Public Health. In addition, there is a need for a well-equipped neonatal intensive care unit close to the delivery room with a well-trained pediatrician to treat critically ill newborns. Further studies at various centers across the country are needed to corroborate our findings and help reduce the incidence of AP and associated morbidity and mortality.
AUTHORS’ CONTRIBUTION
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, data collection, data analysis and interpretation, or all these areas; also they took part in drafting, revising, or critically reviewing the article; and gave final approval of the version to be published
References
- Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand. 2011;90(2):140–9.
- Kovo M, Schreiber L. Placental histopathology and pregnancy outcome in placental abruption. Thrombosis Update. 2021; 5:100087
- Rasmussen S, Irgens LM, Dalaker K. A history of placental dysfunction and risk of placental abruption. Paediatr Perinat Epidemiol. 1999;13(1):9–21.
- Lydon-Rochelle M, Holt VL, Easterling TR, et al. First-birth cesarean and placental abruption or previa at second birth. Obstet Gynecol. 2001;97(5):765–9.
- Sanchez SE, Pacora PN, Farfan JH, et al. Risk factors of abruptio placentae among Peruvian women. Am J Obstet Gynecol. 2006;194(1):225–30.
- Delbaere I, Verstraelen H, Goetgeluk S, et al. Pregnancy outcame in primiparous of advance maternal age. Europ J Obstet Gynecol and Repro Biolog. 2007;135(1):41-46.
- Opara EI, Zaidi J. The interpretation and clinical application of the word 'parity': a survey. BJOG. 2007 Oct ;114(10):1295-7.
- Sangkomkamhang U. The effectiveness of Evidence Based Practice for Reducing Early Postpartum Hemorrhage. Khon Kaen Hospital Med J. 2008;32(2):239-248.
- Ajenifuja KO, Adepiti CA, Ogunniyi SO. Postpartum hemorrhage in teaching hospital in Nigeria. afr health sci 2010; 10(1):71-74
- Dutta DC. Antenatal care, preconception counseling and care. In: Text Book of Obstetrics Including Perinatology and Contraception. 8th ed. India: New central Book Agency;2015; 10-p106
- American College of Obstetricians and Gynecologists. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. 2020 Jun;135(6):e237-60.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011 Jan;34 Suppl 1(Suppl 1):S62-9
- Medina TM, Hill DA. Preterm premature rupture of membranes: diagnosis and management. Am Fam Physician. 2006 Feb 15;73(4):659-64.
- Oyelese Y, Smulian JC. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol.2006;107(4):927–941
- Hiasat MS. The impact of maternal age and parity on the cesarean section rate. JRMS. 2005;12(1):30-34
- MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. Natl Vital Stat Rep. 2012 Aug 28;60(8):1-22
- Hassan AA, Abubaker MS, Radi EA, et al. Education, prenatal care, and poor perinatal outcome in Khartoum, Sudan. International Journal of Gynecology and Obstetrics. 2009;105.(1):66-67.
- Jurdi SR, Jayaram A, Sima AP, et al. Evaluation of a Comprehensive Delivery Room Neonatal Resuscitation and Adaptation Score (NRAS) compared to the Apgar score: a pilot study. Global Pediatric Health. 2015 Jul 31;2:2333794X15598293.
- Khan NY, Bawazeer S. Analysis of perinatal mortality in Aden General Hospital: A hospital-based study from Yemen. Yemen J Med. 2022;1(1):22-26.
- Fisher S. Social inequalities in maternal and perinatal mortality. New Digest. 2008;44:18-26
- Gillissen A, van den Akker T, Caram-Deelder C, et al. Coagulation parameters during the course of severe postpartum hemorrhage: a nationwide retrospective cohort study. Blood Adv. 2018 Oct 09;2(19):2433-2442.
- Rathi M, Rathi SK, Purohit M, et al. Couvelaire uterus. BMJ Case Rep. 2014 Mar 31;2014:bcr2014204211
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2014 Feb 06;370(6):583
- Ghaheh HS, Feizi A, Mousavi M, et al. Risk factors of placental abruption. J Res Med Sci. 2013 May;18(5):422-6
- Akadri AA, Ogunsowo KM, Odelola OI. Abruptio Placenta: A retrospective analysis in a tertiary hospital, Sagamu, Nigeria. Trop J Obstet Gynaecol 2018;35:142-6.
- Nilsen RM, Vollset SE, Rasmussen SA, et al. Folic acid and multivitamin supplement use and risk of placental abruption: a population-based registry study. Am. J. Epidemiol. 2008;167:867-874
- Hossain N, Khan N, Sultana SS, et al. Abruptio placenta and adverse pregnancy outcome. J Pak Med Assoc. 2010 Jun; 60(6):443-6.
- Mukherjee S, Bawa AK, Sharma S, et al. Retrospective study of risk factors and maternal and fetal outcome in patients with abruptio placentae. J Nat Sc Biol Med 2014;5:425-8.
- Dafallah SE, Babikir HE. Risk factors predisposing to abruptio placentae. Maternal and fetal outcome. Saudi Med J. 2004;25(9):1237-1240
- Saeed MU, Rana TA. Fetomaternal outcome in pregnancies complicated with placental abruption. Pakistan Journal of Medical and Health Sciences. 2011;5(1):1-5.
- Barua S, Chakrabarty A, Samanta A, et al. Evaluation of Maternal and Fetal Outcome in Patients of Abruptio Placentae in a Tertiary Care Center, Kolkata: A Descriptive and Observational Study. Asian J Med Sci 2022; 13(8): 208-13.
- Rudakova IS, Shifman EM, Tikhova GP, et al. Hypertensive disorders in pregnancy as a risk factor of premature placental abruption: a systematic review and meta-analysis. Russian Journal of Anaesthesiology and Reanimatology. 2023;2:6–14
- Aktürk E, Emeklioğlu ÇN, Cıngıllıoğlu B, et al. Risk factors and maternal/fetal outcomes of pregnant women with abruptio placenta: a retrospective, descriptive study. J Health Sci Med 2022; 5(6): 1535-1540
- Nankali A, Malek-Khosravi SH, Zangeneh M, et al: Maternal complications associated with severe preeclampsia. The Journal of Obstetrics and Gynecology of India; 2013, 63:112-115.
- Saquib S, Hamza LK, AlSayed A, et al. Prevalence and its feto-maternal outcome in placental abruption: a retrospective study for 5 years from Dubai Hospital. Dubai Medical Journal. 2020;3(1):26-31.
- Sengodan SS, Dhanapal M. Abruptio placenta: a retrospective study on maternal and perinatal outcome. Int J Reprod Contracept Obstet Gynecol 2017;6:4389-92.
- Coleman J, Srofenyo EK, Ofori EK, et al. Maternal and fetal prognosis in abruptio placentae at Korle-Bu Teaching Hospital, Ghana. African Journal of Reproductive Health. 2014;18(4):115-22
- Kaminsky LM, Ananth CV, Prasad V, et al. The influence of maternal cigarette smoking on placental pathology in pregnancies complicated by abruption. Am J Obstet Gynecol. 2007 Sep;197(3):275.e1-5
- Raymond EG, Mills JL. Placental abruption. Maternal risk factors and associated fetal conditions. Acta Obstet Gynecol Scand. 1993;72:633–9.
- Cnattingius S. Maternal age modifies the effect of maternal smoking on intrauterine growth retardation but not on late fetal death and placental abruption. Am J Epidemiol. 1997;145:319–23.
- Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am J Epidemiol. 1996;144:881–9
- Ananth CV, Smulian JC, Vintzileos AM. Incidence of placental abruption in relation to cigarette smoking and hypertensive disorders during pregnancy: a meta-analysis of observational studies. Obstet Gynecol. 1999;93:622–8.
- Bibi S, Ghaffar S, Pir MA, et al. Risk factors and clinical outcome of placental abruption: a retrospective analysis. J Pak Med Assoc. 2009 Oct;59(10):672-4
- Dars S, Sultana F, Akhter N. Abruptio Placentae: Risk Factors and Maternal Outcomes at Tertiary Care Hospital. Journal of Liaquat University of Medical and Health Sciences. 2013;12(3):198-202
- Abbasi RM, Rizwan N, Mumtaz F, et al. Maternal outcome among abruption placentae cases at a University Hospital of Sindh. JLUMHS.2008;7:106-9
- Budde MP, De Lange TE, Dekker GA, et al. Risk factors for placental abruption in a socio-economically disadvantaged region. The journal of maternal-fetal & neonatal medicine. 2007 Jan 1;20(9):687-93.
- Sharma ST. Clinical study of maternal and fetal outcome associated with abruptio placentae. International Journal of Gynaecology. 2019; 11(2): 40-44.
- Jabeen M, Gul F. Abruptio Placentae: Risk factors and perinatal outcome. J Postgrade Med Inst. 2004; 18: 669-76.
- Kapadia LD, Dhrangiya B. Study of Maternal and Perinatal Outcome in 100 Cases of Abruptio Placentae. Int J Med Res Health Sci 2017, 6(7): 84-88
- Tikkanen M, Gissler M, Metsäranta M, et al. Maternal deaths in Finland: focus on placental abruption. Acta Obstet Gynecol Scand. 2009;88(10):1124-7.
- Jadhav K, Kadam M, Lokhande V, et al. Study of Maternal and Foetal Outcome in Abruptio Placentae. International Journal of Medical Science and Clinical Invention. 2021;8(1), 5208–5213.
- Arora R, Devi U, Mujamdar K. Perinatal mortality in antepartum hemorrhage. J Obstetrics and Gynecology. 2001;51(3):102-4.
