Full HTML
Monkeypox virus infection: A clinical review based on the 2022 global outbreak
Raza Ali Akbar1,2, Sofia Ali Raza3, Muhammad Aamir Waheed4
Author Affiliation
1Senior Consultant, epartment of Medicine, Hamad General Hospital, Qatar
2Assistant Professor, Department of Clinical Medicine, Weill Cornell Medicine,
3Student, Department of Secondary Education (Key Stage 4), Doha College, Al-Wajba Campus, Al-Niser Street, Doha, Qatar
4Consultant, Department of Medicine, Hamad General Hospital, Qatar
Abstract
Monkeypox (mpox) is a zoonotic infection caused by an orthopox DNA virus of the family which causes smallpox. The new outbreak of mpox was first reported in Europe in May 2022, which led to cases being reported in nonendemic countries across the globe. The main modes of human-to-human transmission are through bodily secretions, or contact with skin sores. More than 83,000 cases of mpox have been reported globally in 110 affected countries, with 72 confirmed deaths. The predominant mode of transmission is through animal-to-human infected body fluids. The main clinical presentation of patients with mpox is with mucocutaneous manifestation with a range of recorded descriptions of the skin lesions. Most of the patients recover without any medical intervention as mostly the disease is self-limiting. The usual supportive care is needed. Although there is no specific treatment, yet antivirals are used for the treatment of mpox which were originally developed for use in patients with smallpox. Currently, there are two available vaccines which can mitigate the risk of developing mpox. The timely global collaboration between the WHO and different countries has helped to mitigate the public health impact of mpox.
DOI: 10.32677/yjm.v2i2.3801
Keywords: Monkeypox, Orthopox DNA virus, Global outbreak, Zoonotic infection, Endemic
Pages: 75-80
View: 5
Download: 6
DOI URL: https://doi.org/10.32677/yjm.v2i2.3801
Publish Date: 28-09-2023
Full Text
INTRODUCTION
Mpox is a zoonotic infection caused by an orthopox DNA virus of the family which causes smallpox. It has rarely been reported out of Africa until early 2022, when sporadic cases were reported all over the world since April 2022.1 It was first reported in 1970 in Zaire (now called the Democratic Republic of Congo (DRC)) [1].
As of December 22, 2022, more than 83,000 cases have been reported globally in 110 affected countries, with 72 confirmed deaths. [2] Initially, the World Health Organization (WHO) declared mpox an “evolving threat of moderate public health concern” on June 23, 2022. However, due to the rapidly evolving situation, the WHO declared it a global health emergency on July 23, 2022, a designation which was previously used to describe only two other diseases, COVID-19 and Polio. [3] After a series of consultations with global experts, the WHO decided on November 28, 2022, to use the preferred name for monkeypox as ‘Mpox’. The new name will be simultaneously used with the original term of monkeypox for one year until the old name is phased out [4]. Due to significant public health importance, it is imperative that a literature review is carried out on this topic. Hence the epidemiology, pathogenesis, clinical presentation, diagnosis, management, complications and prognosis are reviewed below.
ETIOLOGY AND EPIDEMIOLOGY
Mpox is a zoonotic infection which is caused by an orthopox DNA virus. It belongs to the same family of viruses which causes smallpox (the variola genus) – hence a similarity in appearance and pattern of the rash to smallpox. However, both the human spread and mortality related to mpox are less as compared to smallpox infection. Mpox has two strains identified – clade one (I) with predominance in Congo and clade two (II) in Western Africa. During the global pandemic, two subclades of clade two (II) have been identified as clade IIa and IIb.
It was in the 1950s that mpox was first isolated in Denmark. The mpox virus was identified and isolated from a colony of laboratory monkeys from Singapore. Originally, these monkeys were going to be used for polio virus research at the time [5]. Despite the discovery of the virus in 1950s, the first community spread of mpox was identified nearly two decades later in the 1970s in the DRC. Since then, there have been steady and sporadic reports of infection outbreaks in the African region. Since 2007, there has been an exponential (nearly twenty-times) increase in the incidence of mpox in the DRC as compared to the decade of 1980s [6]. The latest figures from the WHO indicate that mpox is endemic in many sub-Saharan African countries, including Benin, Cameroon, the Central African Republic, the DRC, the Republic of Congo, Gabon, Ghana, Ivory Coast, Liberia, Nigeria, Sierra Leone, and South Sudan – the geographic maps indicating that all these countries are located in the central-to-western region of the sub-Saharan Africa. Reported literature from Africa documented both animal-to-person and person-to-person transmission of mpox. The human-to-human transmission mainly occurs through either infected lesions on the skin or through body fluids. Moreover, surface contact with infected materials can also be a potential source of infection.
PUBLIC HEALTH PERSPECTIVE OF MPOX DURING THE GLOBAL OUTBREAK IN 2022
The new outbreak of mpox was first reported in Europe in May 2022, which then led to cases being reported in nonendemic countries across the globe [7]. The first reported case was from the UK on May 6, 2022, in a patient travelling back to the UK from Nigeria [8]. Despite initial reports of travel-related cases, the vast majority of subsequent cases had no apparent link to travel to endemic areas, leading to the possibility of local transmission of mpox [9-12].
Further cases were reported from Portugal, Spain and other countries in the Western European region and other parts of the world. Since the onset of the global outbreak in May 2022, the cases continued to spread all over the world (including regions like North America, South America, South-east Asia, the Middle East/North Africa and Australia). Hence on July 23, 2022, the WHO declared this outbreak of mpox a public health emergency of international concern.8 In the USA, there have been 29,740 confirmed cases of mpox with 20 deaths (as on December 21, 2022) [13].
TRANSMISSION
The predominant mode of transmission for mpox is animal-to-human through infected animal body fluids. Many types of animals have been found to carry mpox: namely squirrels, rats and monkeys, with rodents being the most likely reservoirs [14].
The human-to-human transmission of mpox became more prominent during the current global outbreak since mid-2022, as majority of cases in Europe and USA were not related to travel to sub-Saharan Africa and/or exposure to animals. The main modes of human-to-human transmission are through bodily secretions, or contact with skin sores, which could be the result of direct sexual contact or non-sexual close intimate contact for a relatively long period of time [14]. A vast majority of cases reported from the non-endemic countries were noted in men who have had sex with men, though any form of intimate contact is a known risk [14,15]. Viral transmission through contact with infected materials (clothing, bed linen etc) or fomites as well as through respiratory secretions is also reported, though prolonged face-to-face contact is required for respiratory spread [14,16]. Maternal-to-fetal transmission of the virus across the placenta (vertical transmission) or during close contact around the time of childbirth is possible which can cause congenital disease, but the overall rates of transmission are not fully known [14].
CLINICAL PRESENTATION
The incubation period for mpox infection is variable between 5 to 13 days, although it can range from as low as 4 days to as high as 21 days [17, 18,19,20]. Mpox has a predominantly biphasic clinical presentation. The initial prodromal phase, which can last up to five days, is characterised by fever, malaise, chills, sweats, lymphadenopathy, and headache. This is almost invariably followed by the cutaneous eruption phase which usually follows 3-4 days after the prodromal phase.
The main clinical presentation of patients with mpox is with mucocutaneous manifestation with a range of recorded descriptions of the skin lesions. The main sites of distribution of these lesions are face, palms, soles, oral mucosa, conjunctivae, genitals and anus or perineal area [21]. The duration of infectiousness is considered to be from the onset of clinical lesions to the time all the lesions have healed (scabbed).
The cutaneous lesions are usually firm and well-circumscribed. They are painful and have central umbilication. The rash typically evolves through stages (macules, papules, vesicles, pustules) before getting crusted and falling off by around day 14 (Figure 1). The mucocutaneous rash has been reportedly indistinguishable from other vesicular eruptions seen in variola (smallpox), varicella and herpes simplex.
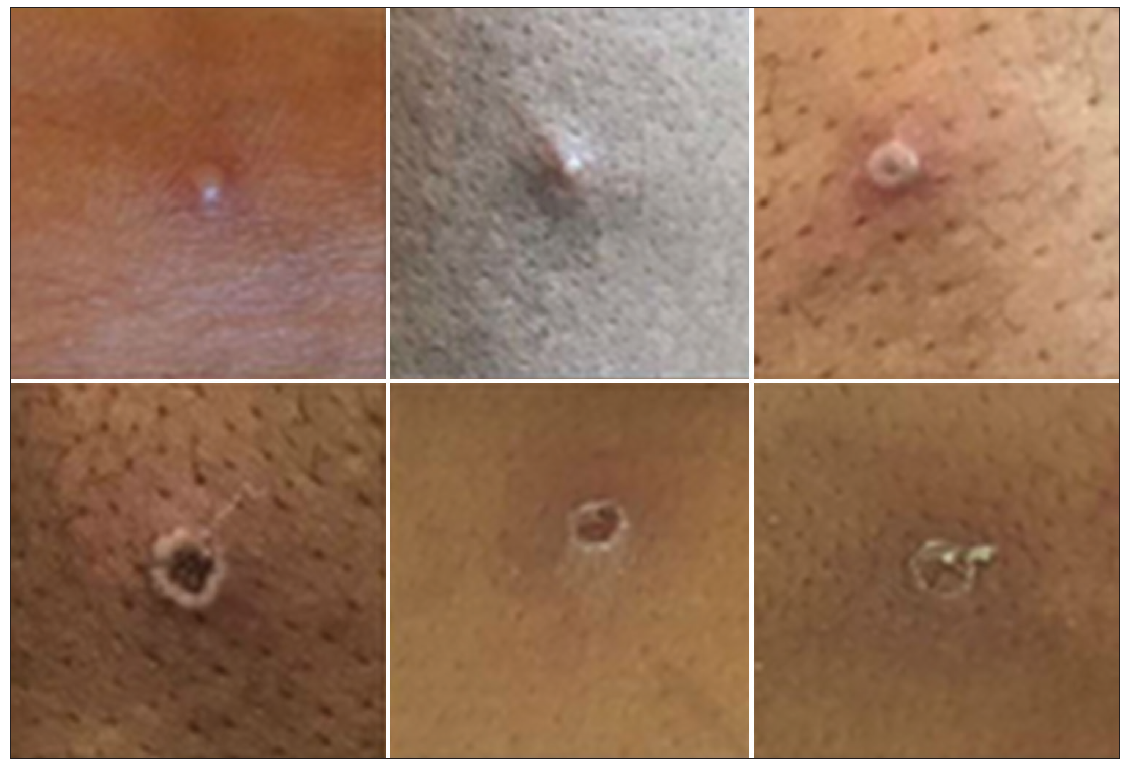
Figure 1: Examples of monkeypox rashes in different stages (photo credit: Courtesy of UK Health Security Agency (Content available under Open Government Licence v3.0.)
Other than mucocutaneous symptoms, the most common reported systemic symptoms are fever, chills, myalgias and lymphadenopathy. Asymptomatic infections seem to be less common as compared to the predominantly symptomatic presentation [22].
DIAGNOSIS
The World Health Organization (WHO) has published case definitions for suspected, probable and confirmed cases of mpox infections (see Table 1) [23]. Diagnostic testing requires viral testing on clinical specimen from the lesion samples for polymerase chain reaction (PCR) or the development of immunoglobulin (Ig)M antibodies.
Table 1: Surveillance case definitions for the current monkeypox outbreak in non‑endemic countries (adopted from the WHO)1

MANAGEMENT
Most of the patients recover without any medical intervention as mostly the disease is self-limiting. The usual supportive care is needed like any other viral prodromal illness, i.e., symptomatic management with antipyretics, analgesics, antiemetics and good hydration and rest. However, patients may require hospitalization in case the symptoms are not controlled, or if the disease leads to any complications.
Patients who should be considered for treatment include those with severe disease (leading to complications like encephalitis, haemorrhagic disease or sepsis), patients with systemic complications (e.g., secondary bacterial skin infection, gastroenteritis, acute kidney injury, pneumonia, etc), patients who may be at high risk of severe disease like with immunocompromised states (primary or secondary due to malignancies, medications, post-transplant etc), patients with a previous history of dermatological conditions like atopic dermatitis or other exfoliative skin conditions and pregnant or breastfeeding women [24].
Though there is no specific treatment approved for mpox virus infections, yet it is deemed prudent to use antivirals developed for use in patients with smallpox. Most experts suggest tecorivimat as the drug of choice, with some suggesting concomitant use of cidofovir especially in patients with severe disease. However, the lack of data on efficacy of cidofovir and the high incidence of nephrotoxicity limits its initial use. It is advisable to involve expert opinion from the infectious diseases specialists &/or local public health officials, whichever the case may be, as per the institutional policies.
Tecovirimat potently inhibits the orthopoxvirus protein which is essential for the viral spread in the infected patient. Both oral and intravenous preparations are available for its use with dosing calculated as per the patient's weight. The recommended duration of treatment with Tecovirimat is 14 days. The side effects are generally mild (nausea, abdominal pain and headache) [25].
VACCINES
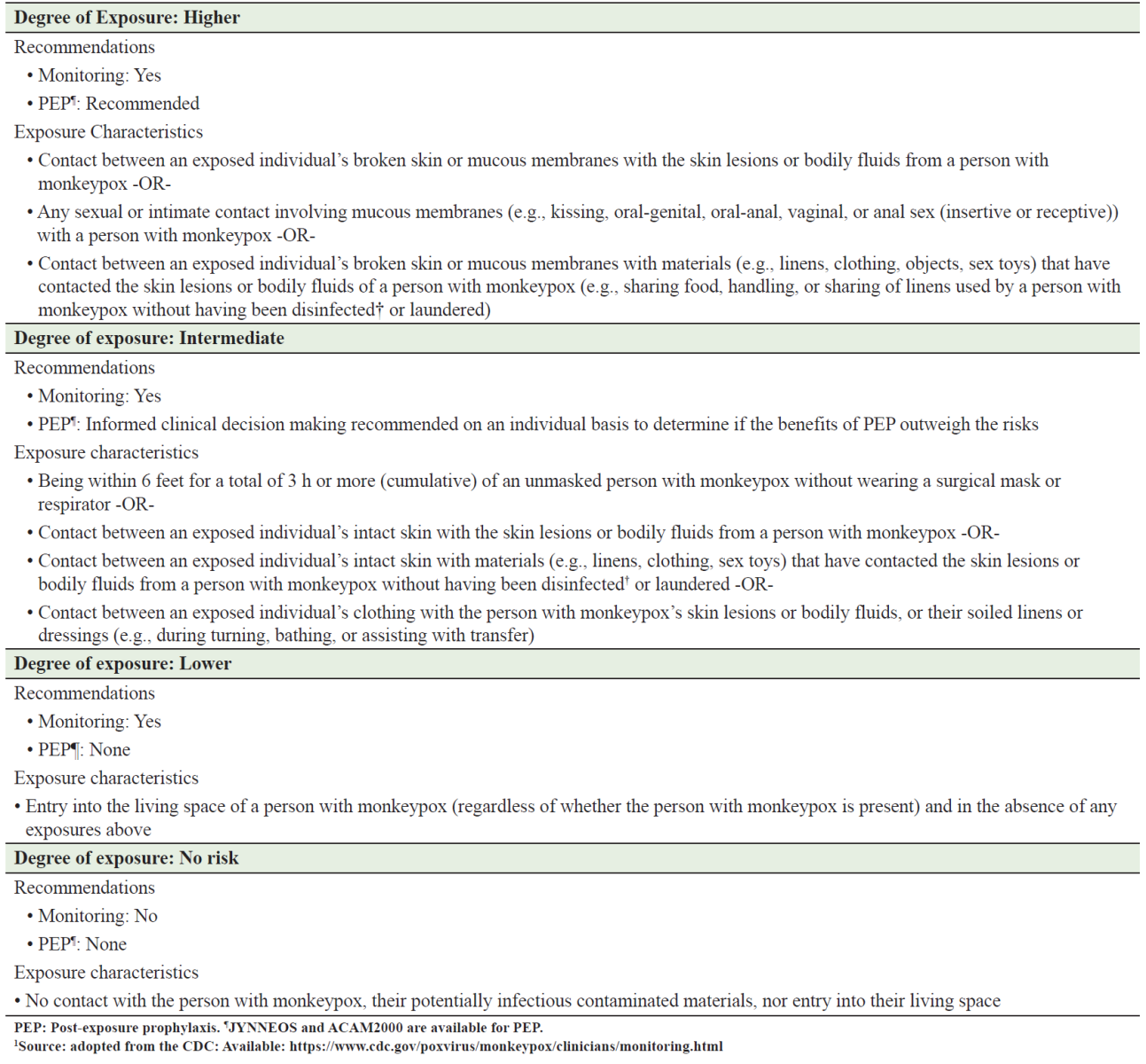
Currently, there are two available vaccines which can mitigate the risk of developing mpox both in pre-exposure and post-exposure phases. The vaccines are the modified vaccinia Ankara (MVA) vaccine (JYNNEOS in the United States, IMVANEX in the European Union, and IMVAMUNE in Canada) and ACAM2000 vaccine [26]. The former (MVA vaccine) is made from a highly attenuated, nonreplicating vaccinia virus administered as two doses, four weeks apart. The ACAM2000 is actually a smallpox vaccine that can only be used in select patients. Generally, MVA vaccine has a much better safety profile than the ACAM 2000 vaccine. In the United States, ACAM2000 is approved for the prevention of smallpox. It can be used for mpox under an expanded access investigational new drug (IND) application through the CDC.26 The selection of high-risk, intermediate-risk and low-risk exposures has been defined by the United States Centers for Disease Control and Prevention (CDC), see table 2 [27].
Table 2: Interim Community Exposure Risk Assessment and Recommendations for Monitoring and Post‑exposure Prophylaxis in Individuals Exposed to monkeypox Virus in a Community Setting (adopted from the CDC)1
COMPLICATIONS
In addition to the classical presentations discussed under the clinical presentation above, patients can also present with complications like proctitis (history of perianal pain, discharge or bleeding especially in patients with a history of anal sex). Other complications/presentations include pharyngitis, tonsillitis, ocular disease (conjunctivitis, keratitis etc) [28]. Other systemic complications include encephalitis, myocarditis, bronchopneumonia, cellulitis and sepsis [29,30].
PROGNOSIS
In a vast majority of cases, mpox is a largely self-limiting illness, which like many other viral infections, resolves in two-to-four weeks without any sequelae. Some patients may develop local or systemic complications as discussed earlier. Clade one (I), the classical form, reportedly has a higher case fatality rate of around 10% [31,32]. In contrast, Clade two (II) variant has a milder disease with less than 1% case fatality rate. The presence of immunocompromised state confers a higher risk for complications and mortality - this specially applies to patients with advances stages of HIV infection [33]
CONCLUSION
The global spread of mpox infection, beyond its accustomed endemic areas, is yet another stark reminder of the abilities of viral strains to defy the usual norms of disease epidemiology and the typical geographical ‘boundaries’. Like Covid-19 pandemic, a concerted global response, led by the WHO, enabled healthcare systems all over the world to share clinical and epidemiological information as well as best practices to everyone’s mutual benefit. This collaboration has invariably identified the key areas of mutual collaboration to mitigate the public health impact of mpox: public health education and awareness (particularly in high-risk population), early detection and prevention and in the strengthening of treatment modalities and vaccination protocols.
AUTHORS’ CONTROBUTION
All authors have made a significant contribution to this work, be it in the conception, implementation, and literature review or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be responsible for all aspects of the work.
References
- J.P. Thornhill, S. Barkati, S. Walmsley, et al., for the SHARE-net Clinical Group. Monkeypox Virus Infection in Humans across 16 Countries — April–June 2022.N Engl J Med 2022; 387:679-691. DOI: 10.1056/NEJMoa2207323
- WHO Health Emergency dashboard. https://extranet.who.int/publicemergency#. (Accessed on December 24, 2022).
- 2022 Monkeypox Outbreak: Global Trends. World Health Organization. https://worldhealthorg.shinyapps.io/mpx_global/. (Accessed on December 24, 2022).
- WHO recommends new name for monkeypox disease. The World Health Organization. Available: https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease. (Accessed on December 24, 2022).
- Von Magnus P, Andersen EK, Petersen KB, et al. A Pox-like Disease in Cynomolgus Monkeys. Acta Pathol Microbiol Scand 1959; 46:156.
- Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A 2010; 107:16262.
- Joint ECDC-WHO Regional Office for Europe Monkeypox Surveillance Bulletin. Available at: https://monkeypoxreport.ecdc.europa.eu/. (Accessed on December 24, 2022).
- Iñigo Martínez J, Gil Montalbán E, Jiménez Bueno S, et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surveill. 2022 Jul;27(27):2200471. doi: 10.2807/1560-7917.ES.2022.27.27.2200471. PMID: 35801519; PMCID: PMC9264731.
- Antinori A, Mazzotta V, Vita S, Carletti F, et al.; INMI Monkeypox Group. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022 Jun;27(22):2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. PMID: 35656836; PMCID: PMC9164671.
- Perez Duque M, Ribeiro S, Martins JV, et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 2022 Jun;27(22):2200424. doi: 10.2807/1560-7917.ES.2022.27.22.2200424. PMID: 35656830; PMCID: PMC9164676.
- UK Health Security Agency. Investigation into monkeypox outbreak in England: technical briefing 2. September 2, 2022 (https://www .gov.uk/government/publications/monkeypox-outbreak-technical-briefings/investigation-into-monkeypox-outbreak-in-england-technical-briefing-2).
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Mpox, Joint Epidemiological overview, 21 December 2022. Available: https://monkeypoxreport.ecdc.europa.eu/
- United States Centers for Disease Control and Prevention. 2022 U.S. Map & Case Count. Available: https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html#print. (Accessed on December 21, 2022).
- World Health Organization. Monkeypox fact sheet. https://www.who.int/news-room/fact-sheets/detail/monkeypox (Accessed on December 21, 2022).
- United States Centers for Disease Control and Prevention. Monkeypox: transmission. https://www.cdc.gov/poxvirus/monkeypox/transmission.html (Accessed on December 21, 2022).
- United States Centers for Disease Control and Prevention. CDC and health partners responding to monkeypox case in the U.S. https://www.cdc.gov/media/releases/2022/s0518-monkeypox-case.html (Accessed on December 21, 2022).
- United States Centers for Disease Control and Prevention. Potential exposure to person with confirmed human monkeypox infection — United States, 2021 https://emergency.cdc.gov/han/2021/han00446.asp. (Accessed on December 21, 2022).
- Brown K, Leggat PA. Human Monkeypox: Current State of Knowledge and Implications for the Future. Trop Med Infect Dis 2016;1:8. doi:10.3390/tropicalmed1010008. pmid:30270859
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox Outbreak - Nine States, May 2022. MMWR Morb Mortal Wkly Rep 2022; 71:764.
- Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med 2004;350:34250. doi:10.1056/ NEJMoa032299. pmid:14736926
- Patel A, Bilinska J, Tam J C H, Da Silva Fontoura D, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series BMJ 2022; 378 :e072410 doi:10.1136/bmj-2022-072410
- Joint ECDC-WHO Regional Office for Europe Monkeypox Surveillance Bulletin. Available at: https://monkeypoxreport.ecdc.europa.eu/ (Accessed on August 10, 2022).
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385. (Accessed on December 21, 2022).
- United States Centers for Disease Control and Prevention. Treatment Information for Healthcare Professionals. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html. (Accessed on December 15, 2022).
- United States Centers for Diseae Control and Prevention. Interim clinical guidance for the treatment of monkeypox. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html#anchor_1655488137245 (Accessed on December 15, 2022).
- United States Centers for Diseae Control and Prevention. Monkeypox and Smallpox Vaccine Guidance. https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html (Accessed on December 15, 2022).
- United States Centers for Diseae Control and Prevention. Monitoring and Risk Assessment for Persons Exposed in the Community. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html (Accessed on November 19, 2022)
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries — April–June 2022. N Engl J Med 2022; 387: 679-91.
- Thornhill JP, Barkati S, Walmsley S, et al., SHARE-net Clinical Group. Monkeypox Virus Infection in Humans across 16 Countries - April-June 2022. N Engl J Med. 2022 Aug 25;387(8):679-691. doi:10.1056/NEJMoa2207323. Epub 2022 Jul 21. PMID: 35866746.
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022 Jul 28;378:e072410. doi: 10.1136/bmj-2022-072410. PMID: 35902115; PMCID: PMC9331915.
- Nalca A, Rimoin AW, Bavari S, et al. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005 Dec 15;41(12):1765-71. doi: 10.1086/498155. Epub 2005 Nov 11. PMID: 16288402.
- Frey SE, Belshe RB. Poxvirus zoonoses--putting pocks into context. N Engl J Med. 2004 Jan 22;350(4):324-7. doi: 10.1056/NEJMp038208. PMID: 14736922.
- Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019 Aug;19(8):872-879. doi: 10.1016/S1473-3099(19)30294-4. Epub 2019 Jul 5. PMID: 31285143; PMCID: PMC9628943.
