Full HTML
Risk factors of intracerebral hemorrhage among the young population in Qatar: Are genetic risk factors involved?
Fahmi Yousef Khan1,2, Hassan J Al Hail3, Hassan Al Hussein3, Musab Ali3, Hassan O Abuzaid4, Dirk Deleu3 Khalid Sharif4, Nada Errayes5, Pankaj Sharma6
Author Affiliation
1Senior consultant, Department of Medicine, Hamad General Hospital, Doha, Qatar
2Weill Cornell Medical College, Department of medicine, Ar-Rayyan, Qatar
3Consultant, Department of Neurology, Hamad General Hospital, Doha, Qatar
4Consultant, Department of medicine, Alkhor Hospital, Alkhor, Qatar
5Medical Fellow, Department of medical education. University of Lincoln, Lincoln, UK
6Senior Consultant, Department of neurology, Institute of Cardiovascular Research, Royal Holloway University of London, London, UK
Abstract
Background: Intracerebral hemorrhage (ICH) has not been widely investigated in young adults. This study aims to describe the risk factors of ICH with a focus on the possible effect of non-modifiable risk factors, such as genetic factors, to assess the ICH outcomes, and to identify the prognostic factors after ICH among young adult patients.
Methods: This prospective observational study was conducted at two hospitals at Hamad Medical Corporation, Qatar, namely Hamad General Hospital and Alkhor Hospital. The study included young patients (16–45 years old) admitted with ICH between January 1, 2015, and December 31, 2018.
Results: We examined 238 consecutive young patients with ICH consisting of 212 (89.1%) males and 26 (10.9%) females. The mean age was 37.8 ± 6.23 years. The most common risk factor found in 187 (78.6%) patients was hypertension, while 19 (8.0%) patients had no obvious risk factors (cryptogenic). The primary site of bleeding was cerebral cortex (lobar) in 107 (44.96%) patients and then basal ganglia in 97 (40.76%) patients. The in-hospital mortality was 19 (8.0%); the National Institutes of Health Stroke Scale > 14 on admission (adjusted OR = 2.06; 95% CI = 1.448–2.938; P < 0.001), Barthel index score ≤ 40 on admission (adjusted OR = 1.09; 95% CI = 1.015–1.178; P = 0.019), and hypertension (adjusted OR = 0.075; 95% CI = 0.008–0.724; P = 0.025) were found to be independent predictors of in-hospital mortality by multivariate analysis. A 1-year follow-up showed mortality in 7 (3.2%) patients and no new events in 139 (63.8%) cases.
Conclusion: Hypertension, smoking, and excessive alcohol consumption are important modifiable risk factors for ICH among young patients in Qatar, requiring early identification and treatment to prevent this dangerous type of stroke. In addition, we recommend conducting further studies focusing on the genetic risk factors of ICH among young adults, particularly those with cryptogenic ICH, to identify whether genetic risk factors are involved.
DOI: 10.32677/yjm.v2i1.3905
Keywords: Intracerebral hemorrhage, in-hospital mortality, risk factors, hypertension, genetics
Pages: 13-17
View: 5
Download: 9
DOI URL: https://doi.org/10.32677/yjm.v2i1.3905
Publish Date: 10-05-2023
Full Text
INTRODUCTION
Spontaneous intracerebral hemorrhage (ICH) is a type of stroke resulting from arterial bleeding directly into the cerebral parenchyma with possible extension to the cerebral ventricles. [1] It represents 10% to 20% of all strokes [2] and is often related to the spontaneous rupture of small blood vessels that have been weakened by chronic arterial hypertension (HTN) or amyloid angiopathy. [2,3] ICH secondary to the rupture of a vascular malformation, coagulopathy, or anticoagulant treatment occurs far less frequently. [2,3,4] ICHs are responsible for 40% of deaths in a month and nearly 54% in a year. [3] Of all strokes among persons aged 15 to 44, approximately 50% are ischemic strokes, 20% are ICH, and 30% are subarachnoid hemorrhages. [5] Notably, in young adult patients, the number of deaths from hemorrhagic strokes is more than double compared to that from ischemic strokes. [6] Since treatment options are limited, ICH prevention is the most effective approach. In young adults, ICH is of concern as it affects the most productive group in the society; however, so far, limited attempts have been made to study the risk factor profiles in these patients [7,8] as most studies describe ICH in the general population. Although a group of risk factors have been identified, which explain an important proportion of the variance in ICH risk, a significant portion of this variation remains unexplained. Heritability estimates based on genome-wide data from unrelated individuals indicate that up to 30% of ICH risk can be explained by common and rare genetic variations. [9]
In Qatar, ICH constitutes 11.6% of all stroke cases, with HTN being the most frequent risk factor..[10] The epidemiology of ICH in Qatar has been well documented; however, most studies have focused on the entire population regardless of age and ethnicity. There is a lack of data on the risk factors of ICH among young patients. Understanding the epidemiology of ICH helps in making treatment decisions toward optimal prevention and treatment of ICH in this age group. Given the increasing physical, emotional, and financial burden inflicted on young ICH patients, we conducted this study with the following objectives: a) to describe the risk factors of ICH with a focus on the possible effect of non-modifiable risk factors, such as genetic factors; b) to assess the ICH outcomes; and c) to identify the prognostic factors after ICH among young adult patients.
methods
Design, population, and setting
This prospective observational study was conducted at Hamad Medical Corporation (HMC), Qatar, particularly Hamad General Hospital and Alkhor Hospital. These hospitals cover all medical and surgical disciplines, including intensive care units. The study included young patients (16–45 years old), locals, and expatriates admitted with suspected spontaneous ICH between January 1, 2015, and December 31, 2018.
Inclusion–exclusion criteria
Patients confirmed with ICH by head computed tomography (CT) scan or magnetic resonance imaging (MRI) were included. ICH diagnosis was based on the international classification of diseases, 10th Revision code I61. [11] Hypertension (HTN) was identified in the following patients: those who were having a history of HTN, were taking antihypertensive medications, or were having a blood pressure of >140/90 mmHg that persisted for at least 14 days after the onset of ICH, and the diagnosis of HTN was confirmed during follow‑up (newly diagnosed HTN). [12] Patients with unidentified risk factors were labeled as cryptogenic ICH; however, if a specific cause was identified by the 1-year follow-up, the cases were re-subtyped. We excluded non-young patients (>45 years old), patients with subarachnoid hemorrhage, patients with a hemorrhagic transformation from ischemic stroke, patients with subdural/epidural hematoma, traumatic ICH, hemorrhages due to tumors, and patients not willing to participate.
Case ascertainment
From January 1, 2015, to December 31, 2018, patients with a first-ever ICH from the total study population were identified prospectively. To identify patients admitted to HMC, daily checks of hospital admissions, discharge records, and death certificates were prepared. There is no private health care for ICH in Qatar. Therefore, our study reflects the likely actual number of young ICH patients.
Sample size
A pilot study of 40 patients was conducted to estimate the prevalence of ICH in young adult patients. We found that ICH among young patients accounts for 5.0% of stroke cases. Assuming a prevalence “P” of 5.0% and based on an absolute precision error (d) of 5% and a type I error (a) of 5%, the calculated sample size was 73. However, for an accurate estimation of the population parameters, 238 patients were included, representing all cases that met the inclusion criteria during the study period. Therefore, a complete enumeration was performed.
Data source and data collection
Each patient in this study was interviewed and examined by one of the authors and was monitored frequently until discharge. We used a data collection sheet to record the following data: demographic data, medical history, stroke risk factors, site of hemorrhage, the National Institutes of Health Stroke Scale (NIHSS), and Barthel index scores upon admission, discharge, and outcomes. The primary outcome of this study was in-hospital mortality, while the secondary outcomes included 1-year mortality and new cardiovascular events.
Data analysis
Data were reported as mean ± standard deviation for quantitative variables, while qualitative variables were reported as numbers and percentages. Univariate analysis was performed to determine the probable risk factors for in-hospital mortality. Variables with p < 0.10 in the univariate analysis were entered in the multiple logistic regression to identify the independent risk factors for in-hospital mortality at p < 0.05. All statistical analyses were performed using SPSS, version 25.0.
Ethical approval
Ethical approval was obtained from the Institutional Review Board at the medical research center, HMC. This study is part of a grant supported by the Qatar national research fund (QNRF#: NPRP 6-068-3-015). Informed consent was obtained from each participant before participation in the study.
RESULTS
Demographical characteristics of patients
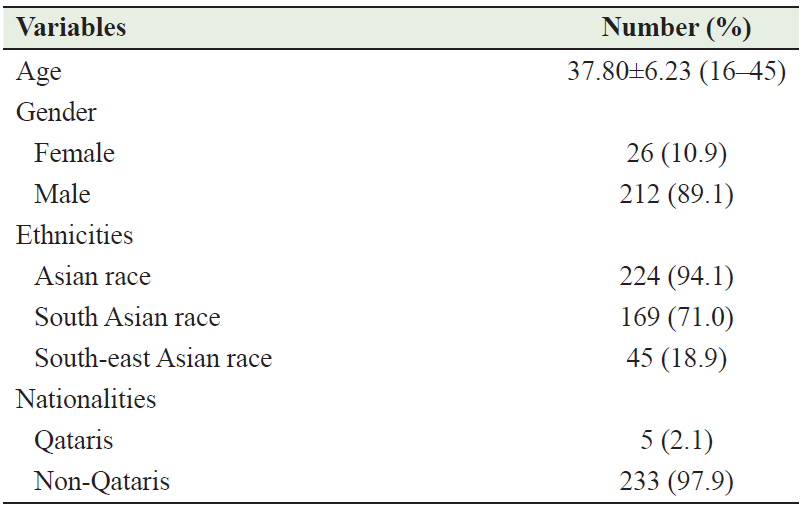
During the study period, 238 consecutive young patients with ICH were identified, representing 30.5% of the total number of ICH cases and 5.4% of total stroke cases admitted during this period. Of the 238 cases, 212 (89.1%) were males and 26 (10.9%) were females. The ages ranged from 16 to 45 years (mean 37.8 ± 6.23). The males were affected more than the females: 212 (89.1%) versus 26 (10.9%) (p < 0.001). A majority of patients were South Asians – 196 (71.0%), followed by 45 (18.9%) Southeast Asians and 24 (10.1%) patients from other ethnicities. Table 1 describes the demographic characteristics of patients involved in this study.
Table 1: Demographic and clinical characteristics of the patients involved in this study
Clinical characteristics
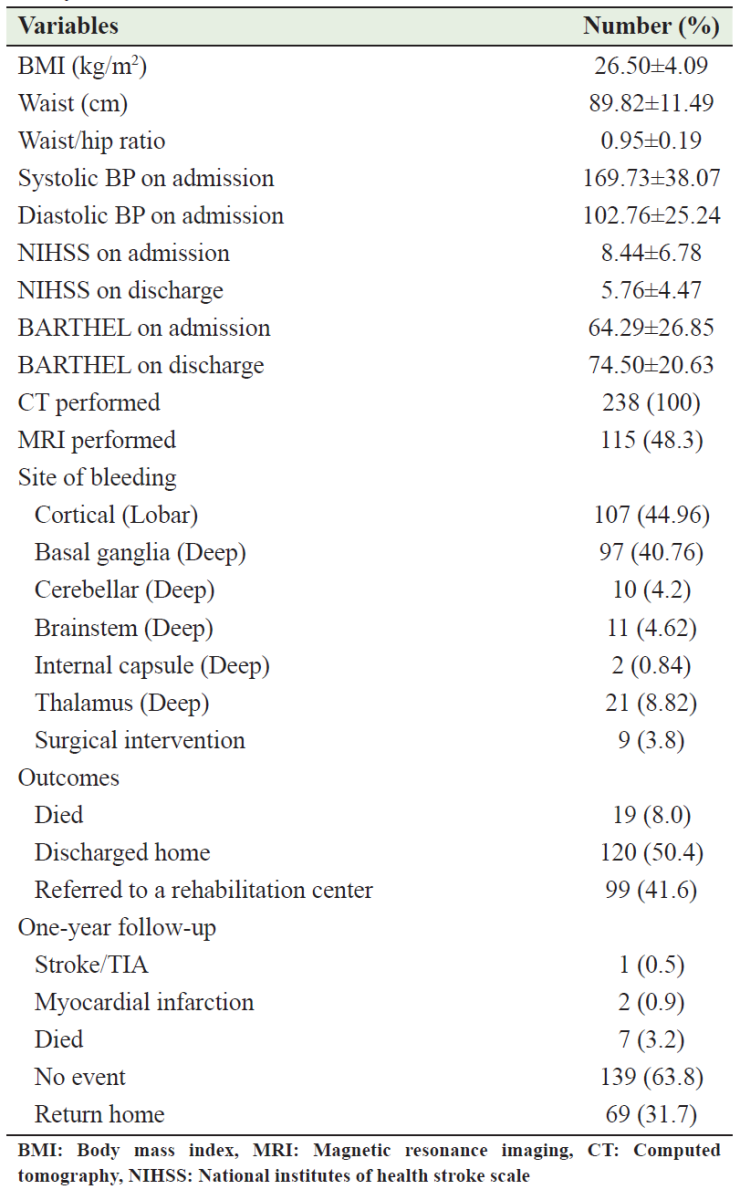
Table 2 summarizes the risk factors of ICH among the cohort of this study. The most common risk factor was HTN found in 187 (78.6%) patients, 12.3% of whom were newly diagnosed; 19 (8.0%) patients had no obvious risk factors (cryptogenic). Of 187 cases with HTN, only 38 (16.0%) patients were taking antihypertensive drugs before the onset of ICH. On admission, the mean NIHSS was 8.44 ± 6.78 and the mean Barthel index score was 64.29 ± 26.85. The primary site of bleeding was cerebral cortex (lobar) in 107 (44.96%) patients and then basal ganglia in 97 (40.76%) patients. In all patients, the diagnosis was confirmed by CT/MRI. Surgical intervention was performed in 9 (3.8%) cases, while the remainder were treated conservatively. Table 3 describes the clinical characteristics of the patients involved in this study.
Table 2: Risk factors of ICH among patients involved in this study
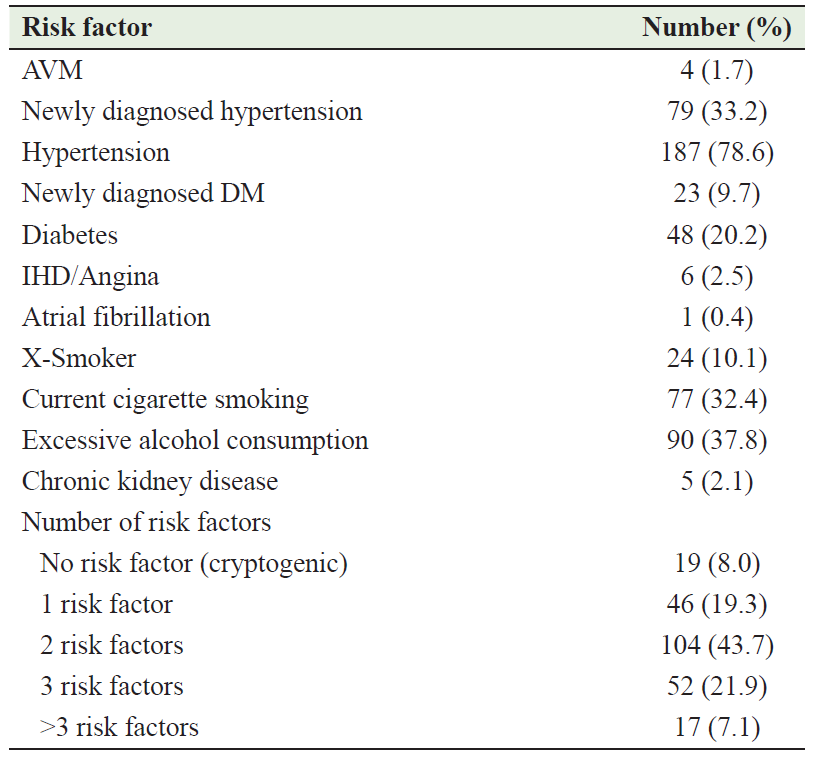
Table 3: Clinical characteristics of the patients involved in this study
Outcomes, predictors of in-hospital mortality, and 1-year follow-up
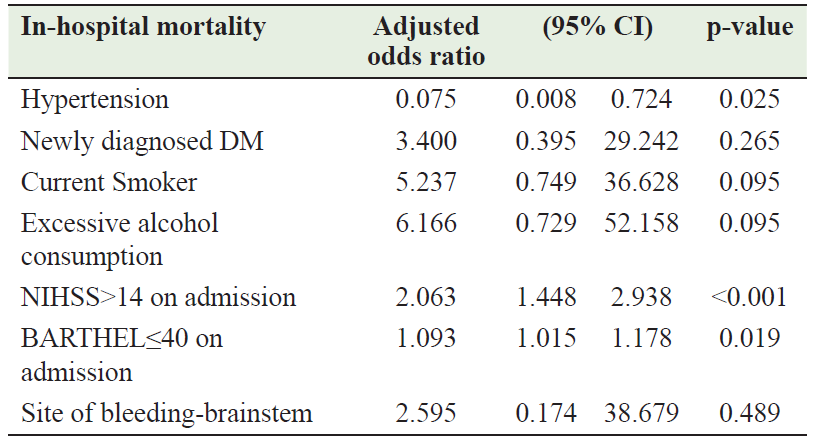
A total of 19 (8.0%) patients died, while 99 (41.6%) were transferred to a rehabilitation center, and 120 (50.4%) were discharged. The univariate analysis found the following variables to be probable predictors of in-hospital mortality: brainstem bleeding, HTN, newly diagnosed DM, alcohol consumption, current smoker, NIHSS > 14 on admission, and Barthel ≤ 40 on admission (Table 4). Only NIHSS > 14 on admission (adjusted OR = 2.06; 95% CI = 1.448–2.938; P < 0.001), Barthel index score ≤ 40 on admission (adjusted OR = 1.09; 95% CI = 1.015–1.178; P = 0.019), and HTN (adjusted OR = 0.075; 95% CI = 0.008–0.724; P = 0.025) were found to be independent predictors of mortality by multivariate logistic regression analysis (Table 5). A 1-year follow-up showed no new events in 139 (63.8%) patients, myocardial infarction in 2 (0.9%) patients, and ischemic stroke in 1 (0.5%) patient, while 7 (3.2%) patients died and 69 (31.7%) patients returned to their home countries (Table 3).
Table 4: Results of univariate analysis of predictors of in‑hospital mortality

Table 5: Results of multivariate analysis of predictors of in‑hospital mortality
DISCUSSION
Stroke is the leading cause of disability worldwide. Compared with ICH in older adults, ICH in young adults has a disproportionately large economic impact by leaving victims disabled before their most productive years. To date, only limited reports have addressed ICH in young patients. To our knowledge, our study is the first to address ICH among young adults in Qatar.
Our study showed important findings that need interpretation and detailed discussion based on existing knowledge. First, we found that most of our patients were non-Qatari males, who were laborers primarily from the Indian subcontinent and far East. This is consistent with the reports from different countries that showed a high incidence of ICH among young males. [5-8] The low incidence of ICH in young women may be related to the female gonadal hormones. [13] In addition, the prevalence of habits such as smoking and alcohol consumption is higher among men than women. [14] The impact of ICH on non-Qatari patients is devastating because they lose their jobs and return to their countries with disabilities that have personal, social, and economic implications. Second, HTN was the most frequent risk factor for ICH, consistent with many studies. [15-17] Interestingly, we found that 9.7% of hypertensive patients were not known to have HTN and presented with ICH as the first manifestation of HTN. This reflects the low level of preventive health‑seeking behavior among this population. Moreover, only 16% of hypertensive patients developed ICH while taking their antihypertensive medications, suggesting drug noncompliance, treatment inertia, or the existence of secondary causes for HTN. These findings call for aggressive preventive measures targeting young patients, such as early detection of HTN through regular checkups, patient education to adhere to antihypertensive medications, and secondary HTN workups if standard drug therapy fails. Since many ICH patients with secondary HTN remain undiagnosed, a high index of clinical suspicion is needed, particularly in young patients with drug-resistant HTN. Not all patients in this study were investigated for secondary HTN. Third, alcohol consumption ranked second on our list of risk factors, which seems odd in a Muslim country such as Qatar, where the Islamic religion forbids the consumption of alcohol. However, this finding can be explained by the fact that most workers in Qatar were not Muslims. Chen et al. [18] found that increased alcohol consumption is associated with ICH at young age. However, the dose-dependent relationship between alcohol consumption and ICH remains unclear. Fourth, smoking was the third most common risk factor in our study. In a previous study in Qatar, [19] smoking was one of the main risk factors found in young adults admitted with ischemic stroke. According to an earlier study, [20] the effect of smoking on ICH has almost the same magnitude as that on ischemic stroke. Finally, our study has reported the presence of more than one risk factor in 72% of the cases, suggesting a multifactorial pathogenic mechanism of ICH. In contrast, no risk factors were identified in 8% of the cases, highlighting the possible role of non-modifiable risk factors such as gender and genetic disorders.
Although several risk factors for ICH have been identified, the frequency varies from country to country. It is unclear why some people with established risk factors develop ICH while others do not. It is also unclear why some people, particularly young patients without such risk factors, develop ICH. These queries also highlight the possible role of non-modifiable risk factors such as gender, race, and genetic disorders.
Some familial aggregation of ICH is noted, and the heritability of ICH risk has been estimated at 44%, indicating that genetic variations contribute substantially to ICH risk and outcome. [4,21] However, it should be noted that despite numerous publications linking genetic variations to ICH risk, it is still possible that there is no clinically significant genetic component specific to ICH. [22] Candidate gene variants reported to be associated with ICH were potentially involved in the following pathways: HTN, vessel wall integrity, lipid metabolism, endothelial dysfunction, inflammation markers, platelet function, and coagulopathy. [4,21,22,23] Therefore, the identification of genetic variants that increase the risk of ICH can improve our understanding of ICH pathogenesis with a potential to both guide preventive strategies and define new therapeutic approaches. [21,22,23] We recommend conducting further studies focusing on genetic risk factors for ICH particularly among young adults, as there is a lack of such studies among this age group.
In-hospital mortality among young patients with ICH ranged from 12.5% to 34.1%. [24] In our study, the in-hospital mortality was 8%, which is lower than the range previously mentioned. Although ICH was considered a risk factor for in-hospital mortality [25], few studies have reported risk factors associated with early mortality in young adult patients with ICH. Zhou et al. [16] found that only infratentorial hemorrhage (P = 0.003) and intraventricular extension (P = 0.003) were significant risk factors for early mortality. While Koivunen et al. [26] found NIHSS > 14, infratentorial hemorrhage, hydrocephalus, multiple hemorrhages, brain herniation, and hematoma evacuation are significant risk factors for 3-month mortality in young adults after ICH. Our study found NIHSS > 14 on admission, presence of HTN, and Barthel index score ≤ 40 on admission as risk factors for in-hospital mortality.
There are a few limitations to our study. First, alcohol consumption was based on the number of drinks rather than the exact amount of alcohol consumed. Second, less than half of our patients (48.3%) underwent MRI and none underwent angiographic imaging. This may have led to an underestimation of structural causes underlying ICH. Strengths of our study include its large sample size, and the fact that although our study is hospital based, it can be considered nearly a population-based study because there is no private health care for ICH in Qatar other than HMC.
CONCLUSION
To conclude, HTN, smoking, and excessive alcohol consumption are important modifiable risk factors for ICH among young patients in Qatar, requiring early identification and treatment to prevent this dangerous type of stroke. Most hypertensive patients were either untreated or did not meet treatment goals. Improved strategies for the detection and treatment of HTN are needed for the primary prevention of ICH. In addition, we recommend conducting further studies focusing on genetic risk factors for ICH among young adults, particularly those with cryptogenic ICH, to identify whether genetic risk factors are involved.
AUTHORS’ CONTRIBUTION
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; and gave final approval of the version to be published.
References
- Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450-60
- Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8(4):355-69..
- An SJ, Kim TJ, Yoon BW. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J Stroke. 2017;19(1):3-10.
- Carpenter AM, Singh IP, Gandhi CD, et al. Genetic risk factors for spontaneous intracerebral haemorrhage. Nat Rev Neurol. 2016 Jan;12(1):40-9
- Jacobs BS, Boden-Albala B, Lin IF, et al. Stroke in the young in the northern Manhattan stroke study. Stroke 2002;33:2789–2793.
- Krishnamurthi RV, Moran AE, Feigin VL, et al. Stroke Prevalence, Mortality and Disability-Adjusted Life Years in Adults Aged 20-64 Years in 1990-2013: Data from the Global Burden of Disease 2013 Study. Neuroepidemiology. 2015;45(3):190-202.
- Ruíz-Sandoval JL, Cantú C, Barinagarrementeria F. Intracerebral hemorrhage in young people: analysis of risk factors, location, causes, and prognosis. Stroke. 1999;30(3):537-41.
- Ruiz-Sandoval JL, Romero-Vargas S, Chiquete E, et al. Hypertensive intracerebral hemorrhage in young people: previously unnoticed age-related clinical differences. Stroke. 2006;37(12):2946-50.
- Devan WJ, Falcone GJ, Anderson CD, et al.; International Stroke Genetics Consortium. Heritability estimates identify a substantial genetic contribution to risk and outcome of intracerebral hemorrhage.Stroke. 2013; 44:1578–1583.
- Imam YZ, Kamran S, Saqqur M, et al. Stroke in the adult Qatari population (Q-stroke) a hospital-based retrospective cohort study. PLoS One. 2020;15(9):e0238865.
- Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064‑89.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021‑104.
- Gokhale S., Caplan LR., James ML. Sex differences in incidence, pathophysiology, and outcome of primary intracerebral hemorrhage, Stroke., 2015, 46, 886-892.
- Zia E, Engström G, Svensson PJ, et al. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage, Stroke., 2009, 40, 3567-3573.
- Bi Q, Wang L, Li X, et al. Risk factors and treatment of stroke in Chinese young adults. Neurol Res 2010;32(4):366–70.
- Zhou HX, Hao N, Xu XL. Related Factors of Early Mortality in Young Adults with Cerebral Hemorrhage. Open Med (Wars). 2018;13:214-220.
- Chen CY, Lin PT, Wang YH, et al. Etiology and risk factors of intracranial hemorrhage and ischemic stroke in young adults. J Chin Med Assoc. 2021;84(10):930-936.
- Chen CJ, Brown WM, Moomaw CJ, et al. Alcohol use and risk of intracerebral hemorrhage. Neurology. 2017;88(21):2043-2051.
- Khan FY. Risk factors of young ischemic stroke in Qatar. Clin Neurol Neurosurg 2007; 109(9): 770-3.
- Kurth T, Kase CS, Berger K, et al. Smoking and the risk of hemorrhagic stroke in men. Stroke. 2003;34(5):1151-5.
- Guo H, You M, Wu J, et al. Genetics of Spontaneous Intracerebral Hemorrhage: Risk and Outcome. Front Neurosci. 2022;16:874962.
- Chen YC, Chang KH, Chen CM. Genetic Polymorphisms Associated with Spontaneous Intracerebral Hemorrhage. Int J Mol Sci. 2018;19(12):3879.
- Falcone GJ, Woo D. Genetics of Spontaneous Intracerebral Hemorrhage. Stroke. 2017;48(12):3420-3424.
- Tatlisumak T, Cucchiara B, Kuroda S, et al. Nontraumatic intracerebral hemorrhage in young adults. Nat Rev Neurol. 2018;14: 237–250.
- Basamed JM. Risk factors and outcomes of stroke in a tertiary hospital in Hadhramout Governorate, Yemen. Yemen J Med. 2022;1(2):69-73
- Koivunen RJ, Satopää J, Haapaniemi E, et al. Predictors of early mortality in young adults after intracerebral hemorrhage. Stroke. 2014;45(8):2454-6.
