Full HTML
A case of cyclophosphamide-induced posterior reversible encephalopathy syndrome: Is it dose-related side effect?
Theeb Osama Sulaiman, Ahmed K A Yasin, Abdellatif Ismail
Author Affiliation
From 1Consultant,
2Resident, Department of Medicine, Hamad General Hospital, Doha, Qatar
Abstract
We reported a case of cyclophosphamide (CYP)-induced posterior reversible encephalopathy syndrome (PRES) in a 26-year-old previously healthy male patient who was presented to the emergency department with a history of fever, shortness of breath, and hemoptysis. After extensive investigations, including bronchoscopy and autoimmune screening, he was diagnosed with renalpulmonary syndrome. The diagnosis of CYP-related PRES was based on the development of neurological clinical picture supported by magnetic resonance imaging findings. The dose of CYP was decreased to 75 mg/day, and the patient’s symptoms improved after 3 days.
DOI: 10.32677/yjm.v1i2.3684
Keywords: Antineutrophil cytoplasmic antibody, Cyclophosphamide, Posterior reversible encephalopathy syndrome, Renalpulmonary disease
Pages: 103-107
View: 6
Download: 13
DOI URL: https://doi.org/10.32677/yjm.v1i2.3684
Publish Date: 29-03-2025
Full Text
Posterior reversible encephalopathy syndrome (PRES) is a neurological disease with clinical and radiological manifestation; it was first described by Hinchey et al. in 1996 [1]. The clinical presentation can be varied, including headache, seizure, visual impairment, altered level of consciousness, or coma. Most cases of PRES are secondary, mainly to autoimmune diseases, uncontrolled hypertension, preeclampsia, renal failure, and drugs (primarily immunosuppression and cytotoxic medications) [2]. Cyclophosphamide (CYP) is an immunosuppressive drug that is commonly used for the treatment of different autoimmune diseases and malignancies. It acts by inhibiting cellular and humoral immunity [3]. The most commonly reported side effects include bone marrow suppression, liver injury, cardiotoxicity, stomatitis, and hemorrhagic cystitis.
PRES secondary to CYP has been reported in a few articles; most of these patients received intravenous CYP and improved after blood pressure (BP) control and withdrawal of CYP. Here, we report a case of PRES that occurred in a patient with anti-glomerular basement membrane (GBM) disease after the administration of oral CYP. The patient’s symptoms resolved completely after CYP dose decrease without any serious complications. Our case emphasizes that CYP-related PRES is dose dependent.
CASE REPORT
On August 15, 2021, a young 26-year-old previously healthy male was presented to the emergency department with a history of fever, shortness of breath, and hemoptysis. The patient stated that he had had four to five episodes of hemoptysis per day with an estimated blood loss of 40 ml for the past 1 week. There were no similar complaints in the past nor there were any history of chest pain, facial swelling, or hematuria. There was also no history of joint pain, bruises, petechial rashes, photosensitivity, or photophobia.
At presentation, the patient’s vitals showed BP of 125/74 mmHg, heart rate of 95 beat/min, temperature of 37.8°C, and oxygen saturation of 99% with 2 L of oxygen through a nasal cannula. Cardiac examination demonstrated tachycardia, a normal S1 and S2 with no murmur or rub; chest examination revealed diffuse coarse crackles in both lungs; other physical examination was unremarkable. Laboratory investigations showed hemoglobin of 5.6 g/dl, white blood cell count 7900/µL, and platelets count of 307,000/µL. Serum creatinine was 641 µmol/L; urea 15.3 mmol/L; potassium 4.5 mmol/L; sodium 135 mmol/L; bicarbonate 18 m Eq/L; C-reactive protein 27.9 mg/L; alanine transaminase 307 U/L; aspartate transaminase 300 U/L; spot urine protein/creatinine ratio was high (424 mg/mmol creatinine); and microscopic urine analysis showed 84 RBCs/high-power field with granular casts.
The patient was admitted to the medical intensive care unit, and a bedside bronchoscopy revealed pulmonary hemorrhage. The patient was diagnosed with renal- pulmonary syndrome, and the glomerulonephritis workup was significant for positive anti-GBM with titers of 550 U/ml, but negative for antinuclear antibodies, antineutrophil cytoplasmic antibodies, and normal levels of C3, C4.
The patient was commenced on IV methylprednisolone 1 g daily for 5 days, followed by oral prednisolone 60 mg daily, and hemodialysis was initiated. Renal biopsy was done under ultrasound guidance and histopathology showed crescentic glomerulonephritis, which is consistent with anti-GBM disease. On day 4, plasmapheresis was started, and on day 9, the patient was started on CYP 1 mg/kg. The patient improved clinically, and the anti-GBM titers dropped to 4.7 U/ml.
However, 21 days after starting CYP, the patient suddenly developed an acute headache, mainly periorbital with a blurring of vision and had one episode of vomiting. He was found to have an elevated BP of 170/84 mmHg: Rest of the vital signs were within normal limits. His pupils were regular and reactive to light as normal. Rest of the neurological examination was unremarkable. Head computed tomography scan revealed small, ill-defined hypodense lesions in the bilateral occipital cortical and subcortical regions. Magnetic resonance imaging (MRI) was performed on the same day and it showed bilateral parietooccipital lesions, more prominent on the left side, cortical and subcortical areas of abnormally high signal intensity in T2/FLAIR weighted images (Fig. 1a-d). The dose of CYP was decreased to 75 mg/day, and the patient’s symptoms improved after 3 days.
DISCUSSION
A wide variety of conditions have been associated with the PRES, including bone marrow or stem cell transplantation, uncontrolled hypertension, autoimmune diseases, renal failure, and immunosuppressive medication. Although many meditations such as chemotherapy and cytotoxic agents have been described in association with PRES, its association with CYP is rare. A total of 16 case reports diagnosed with CYP-induced PRES were reviewed [3-18].
Table 1 summarizes the clinical characteristics of the reported cases of CYP-induced PRES, including ours. As noted, the most common presenting complaint was seizures (94.1%) followed by visual symptoms (64.7%) and headache (41%). Nausea, vomiting, and focal neurologic deficits have also been observed in the reported cases.
Interestingly, our patient was the only case who was presented without a history of seizures and our patient developed PRES after 3 weeks of CYP administration. As noted in Table 1, most patients developed symptoms late up to 1–3 months [4,5,8,17] post-CYP initiation. Furthermore, 70.5% (n=12) of these patients received intravenous CYP, while only five patients, including ours (29.4%), received oral prescription.
Although the exact pathogenesis of CYP-induced PRES is poorly understood, two hypotheses have been postulated to describe this association. The first theory described that severe hypertension can lead to failed autoregulation, subsequent hyperperfusion with endothelial injury and vasogenic edema. The second theory postulated that vasoconstriction and hypoperfusion that occur in PRES can lead to brain ischemia and subsequent vasogenic edema [19]. The first hypothesis is currently more acceptable as it is based on the fact that BP is frequently elevated at the diagnosis of PRES and treating hypertension is associated with improvement of PRES symptoms. Review of the literatures showed that the mean peak systolic and diastolic
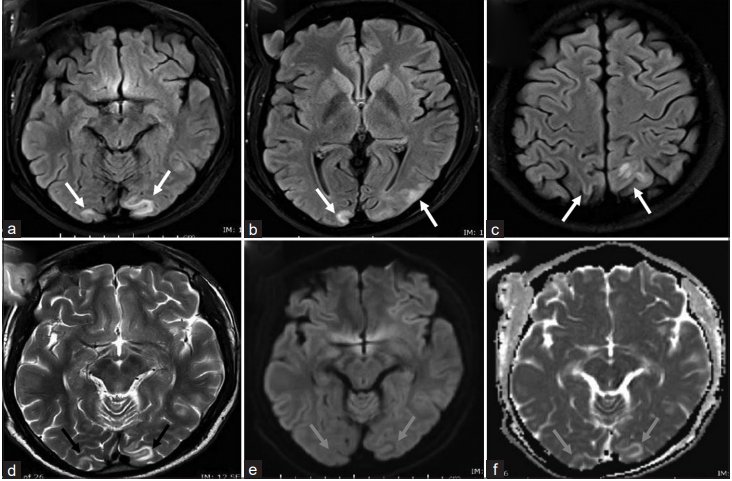
Figure 1: Magnetic resonance imaging of the brain. (a-c) FLAIR images show areas of high signal intensity (white arrows) involving the cortical and subcortical white matter regions of the bilateral occipital and parietal lobes more prominent on the left side. (d) T2 sequence shows corresponding occipital high signal intensity (black arrows). (e, f) Diffusion-weighted imaging and apparent diffusion coefficient sequences show no diffusion restriction (blue arrows) in the occipital lob correspond to the regions of high T2/FLAIR signal intensity which represent vasogenic edema. These findings are typical of PRES syndrome
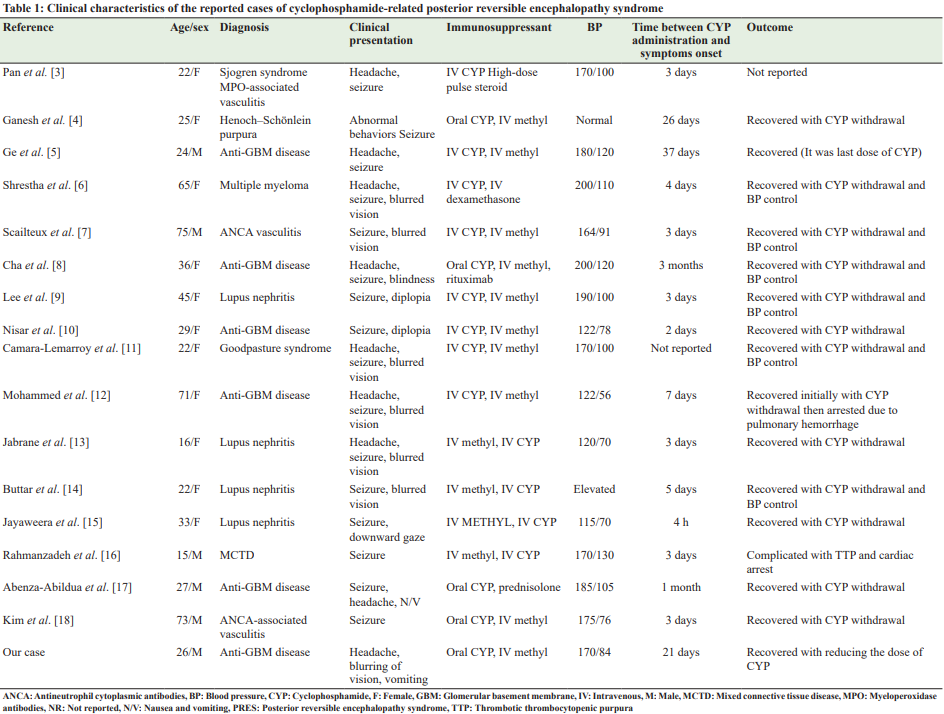
BP at the time of PRES presentation was 199 and 109 mmHg, respectively, and only 10–30% of the patients have normal BP [1,20]. These data are close to the findings mentioned in Table 1, where 12 patients (70.5%), including ours, were hypertensive at time of diagnosis and normal BP was recorded in the other 5 patients (29.4%).
Head MRI is the imaging of choice when PRES is suspected; the findings can include hyperintensities areas on T2 FLAIR images that are not usually visible on diffusion-weighted imaging [21,22]. It is crucial to recognize PRES early to provide adequate management and to avoid serious consequences that include cerebral infarction and death. Treatment of PRES is mainly supportive and consists of management of the underlying disease, antihypertensive agents for the control of high BP, antiepileptic medications to control seizures if present, and elimination or reduction of the dosage of any offending agents such as immunosuppressive medications [13]. In Table 1, all patients who had recovered were treated by eliminating CYP, except in our patient, who achieved complete recovery by decreasing the dose of CYP from 100mg to 75 mg. Improvement of PRES symptoms by only decreasing the dose of immunosuppressive medication was noted with some immunosuppressive medication such as tacrolimus and cyclosporine [23], where a positive correlation was noted between the dose of the offending agent and the neurological/radiological manifestations of PRES, and symptoms improvement was noted on the tapering off or reduction of the dose of these drugs. Almost 10% of patients with PRES will have residual neurological deficits, and around 90% will achieve full neurologic recovery without any deficits, whereas the mortality rate ranges from 3% to 6%, and its usually related to brain hemorrhage, posterior fossa edema with brainstem compression, diffuse cerebral edema, and increased intracranial pressure [24]. The cases reviewed in Table 1 showed that 14 patients (82.3%) recovered, 2 patients (11.7%) died, and the outcome was not reported in one patient. Among the two fatalities was a 71-year-old woman with anti-GBM disease who initially recovered with CYP withdrawal and then developed a pulmonary hemorrhage [12]. A second case was a 15-year-old male patient with mixed connective tissue disease whose hospitalization was complicated by thrombotic thrombocytopenic purpura and cardiac arrest [16].
CONCLUSION
PRES is a recognized side effect of oral CYP. Physicians should carefully monitor neurologic symptoms after oral administration of this drug in patients with renal- pulmonary diseases. Our case emphasizes that CYP-related PRES is dose dependent and can be treated by CYP dose adjustment.
AUTHORS’ CONTRIBUTION
Sulaiman contributed to writing the manuscript and reviewing the literature. Yasin AKA contributed to developing the work idea composing and revising the manuscript. Ismail A contributed to composing and revising the manuscript. All authors read the manuscript and agree to its publication.
References
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494-500.
2. Hinduja A. Posterior reversible encephalopathy syndrome: Clinical features and outcome. Front Neurol 2020;11:71.
3. Pan D, Sabharwal B, Vallejo F. Posterior reversible encephalopathy syndrome secondary to cyclophosphamide in the treatment of pulmonary renal syndrome. Chest 2017;152:A367.
4. Ganesh K, Nair RR, Kurian G, et al. Posterior reversible encephalopathy syndrome in kidney disease. Kidney Int Rep 2017;3:502-7.
5. Ge YT, Liao JL, Liang W, et al. Anti-glomerular basement membrane disease combined with IgA nephropathy complicated with reversible posterior leukoencephalopathy syndrome: An unusual case. Am J Case Rep 2015;16:849-53.
6. Shrestha GS, Nepal G, Basnet B, et al. Cyclophosphamide-induced posterior reversible encephalopathy syndrome: A case report. Neurol Clin Neurosci 2021;9:349-52.
7. Scailteux LM, Hudier L, Renaudineau E, et al. Posterior reversible encephalopathy syndrome after a first injection of cyclophosphamide: A case report. J Pharmacovigil 2015;3:163.
8. Cha B, Kim DY, Jang H, et al. Unusual case of posterior reversible encephalopathy syndrome in a patient with anti-glomerular basement membrane antibody glomerulonephritis: A case report and review of the literature. Electrolyte Blood Press 2017;15:12-6.
9. Lee CH, Lee YM, Ahn SH, et al. Cyclophosphamide-induced posterior reversible encephalopathy syndrome in a patient with lupus nephritis. J Rheum Dis 2013;20:103-7.
10. Nisar T, Alchaki AR, Feinstein E. A rare case of cyclophosphamide-induced posterior reversible encephalopathy syndrome in a patient with antiGBM vasculitis, and review of current literature. Case Rep Neurol Med 2019;2019:2418597.
11. Camara-Lemarroy CR, Cruz-Moreno MA, Gamboa-Sarquis RN, et al. Goodpasture syndrome and posterior reversible encephalopathy syndrome. J Neurol Sci 2015;354:135-7.
12. Mohammed E, Ramrattan A, Santoriello D. Posterior reversible encephalopathy syndrome secondary to cyclophosphamide. Caribb Med J 2020;82:1-4.
13. Jabrane M, Ait Lahcen Z, Fadili W, et al. A case of PRES in an active lupus nephritis patient after treatment of corticosteroid and cyclophosphamide. Rheumatol Int 2015;35:935-8.
14. Buttar C, Lakhdar S, Castillo FC, et al. Cyclophosphamide induced posterior reversible encephalopathy syndrome (PRES) in a patient with lupus nephritis. Clin Med Rev Case Rep 2021;8:354.
15. Jayaweera JL, Withana MR, Dalpatadu CK, et al. Cyclophosphamideinduced posterior reversible encephalopathy syndrome (PRES): A case report. J Med Case Rep 2014;8:442.
16. Rahmanzadeh R, Rahmanzade R, Zabihiyeganeh M. Posterior reversible encephalopathy syndrome in a patient with mixed connective tissue disease: A case report. J Med Case Rep 2016;10:145.
17. Abenza-Abildua MJ, Fuentes B, Diaz D, et al. Cyclophosphamide-induced reversible posterior leukoencephalopathy syndrome. BMJ Case Rep 2009;2009:bcr07.2008.0467.
18. Kim Y, Kwak J, Jung S, et al. Oral cyclophosphamide-induced posterior reversible encephalopathy syndrome in a patient with ANCA-associated vasculitis: A case report. World J Clin Cases 2021;9:6130-7.
19. Bartynski WS. Posterior reversible encephalopathy syndrome, Part 2: Controversies surrounding pathophysiology of vasogenic edema. Am J Neuroradiol 2008;29:1043-9.
20. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: Associated clinical and radiologic findings. Mayo Clin Proc 2010;85:427-32.
21. Camara-Lemarroy CR, Lara-Campos JG, Perez-Contreras E, et al. Takayasu’s arteritis and posterior reversible encephalopathy syndrome: A case-based review. Clin Rheumatol 2013;32:409-15.
22. Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: Prognostic utility of quantitative diffusionweighted MR images. Am J Neuroradiol 2002;23:1038-48.
23. Wu Q, Marescaux C, Wolff V, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol 2010;64:169-77.
24. Kalaiselvan MS, Renuka MK, Arunkumar AS. Clinical features and outcomes of patients with posterior reversible encephalopathy syndrome. Indian J Crit Care Med 2017;21:453-6.
