Full HTML
A case of acute ischemic stroke in moyamoya syndrome associated with Graves’ disease: Is there a role for anti-dsDNA antibodies?
Sumaira Rafique, Koutaiba Rida Obaid, Islam Ahmed Hassan
Author Affiliation
From 1Consultant,
2Resident, Department of Medicine,
3Consultant Radiologist, Department of Radiology, Hamad General Hospital, Doha, Qatar
Abstract
The coexistence of Moyamoya syndrome (MMS) and Graves’ disease (GD) is uncommon. Here, we report a case of a 41-year-old Filipino female, who presented with thyrotoxicosis and acute ischemic stroke. Based on her clinical presentation, cerebral computed tomography angiography, and thyroid function tests, she was diagnosed with MMS and GD. Her Burch-Wartofsky point scale score was 30, suggesting an impending thyroid storm. Antithyroid therapy was started with her neurological status deterioration initially, but after controlling the thyroid storm, the patient’s neurological status stabilized. She remained stable till she travelled to her country. We hypothesized that MMS in a patient with GD is mediated through anti-dsDNA antibodies, by altering key biological mechanisms, that is, inflammation, neutrophil extracellular traps, and apoptosis that drive a distinctive and coordinated immune and vascular activation. To the best of our knowledge, this is the first case of MMS associated with GD reported in Qatar.
DOI: 10.32677/yjm.v1i2.3386
Keywords: Acute stroke, Graves’ disease, Moyamoya disease, Moyamoya syndrome, Thyrotoxicosis
Pages: 93-96
View: 5
Download: 13
DOI URL: https://doi.org/10.32677/yjm.v1i2.3386
Publish Date: 29-03-2025
Full Text
Moyamoya disease (MMD) is a disorder characterized by bilateral stenosis or occlusion of the terminal internal carotid artery (ICA) and proximal portion of the anterior and middle cerebral arteries and the formation of collaterals called moyamoya vessels to compensate for the steno-occlusion [1]. The term MMD is reserved for cases in which the intracranial vascular changes are primary and not associated with any known condition, while the term Moyamoya syndrome (MMS) is used for intracranial vascular changes that occur in association with other conditions, such as postcranial radiotherapy, neurofibromatosis Type 1, Down syndrome, and sickle cell anemia [1,2]. The diagnosis of both MMD and MMS is based on characteristic smoky appearance on angiography like a puff of smoke in the air [1,2].
Graves’ disease (GD) is an autoimmune disorder in which autoantibodies against the thyroid-stimulating hormone (TSH) receptor induce continuous stimulation of the thyroid gland without negative feedback, leading to hyperthyroidism. Conventionally, GD is characterized by hyperthyroidism, diffuse goiter, ophthalmopathy, and dermatopathy. Additional tests such as antibodies to thyroglobulin, thyroid peroxidase, and the TSH receptor provide evidence supporting GD rather than confirming the diagnosis [3,4]. MMS associated with GD is assumed to be rarely reported; however, this clinical entity has been described increasingly in scientific literature in the past few years [4-16]. In this report, we present a case of acute cerebral infarction in a patient with MMS, which appeared to be aggravated by thyrotoxicosis. We aimed to reveal the clinical features, pathogenesis, and treatment effects in MMS patients with concurrent GD.
CASE REPORT
A 41-year-old Filipino woman presented with sudden onset left-sided weakness and slurred speech, which she noticed after waking up. On further questioning, she admitted that she had experienced palpitations, tremors, profuse sweating, heat intolerance, and a weight loss of 17 kg in the past year. Her medical history was relevant for diabetes mellitus diagnosed 4 years ago, for which she was on metformin 500 mg twice daily. On arrival to the emergency department, she was agitated and anxious but alert, conscious, and oriented to time, place, and person. Her pulse was 138 beat/min, blood pressure was 174/104 mm Hg, and her temperature was 36.8°C. A general physical examination showed exophthalmos, lid lag, lid retraction, and a palpable thyroid goiter, which was soft and uniform in character. Neurological examination revealed leftsided weakness with a power of 2/5 in the left lower limb and a power of 4/5 in the left upper limb. There was left-sided hyperreflexia, decreased sensation in the left side, and leftsided facial droop.
Initial laboratory test revealed marked thyrotoxic state (TSH of <0.01 mIU/L, Free T3 of 30.9 pmol/L, and Free T4 of 98.5 pmol/L), and an urgent electrocardiogram showed sinus tachycardia. Initial head computed tomography (CT) scan without contrast (Fig. 1a) showed focal area of hypodensity seen in the right anterior frontal region, likely representing recent infarction along the right anterior carotid artery (ACA) territory. Head CT angiogram with contrast (Fig. 1b) showed multifocal stenosis affecting small and medium sized arteries. The patient was evaluated by the neurology team, who proposed a differential list including fibromuscular dysplasia, MMD, and central nervous system vasculitis. The patient subsequently underwent contrastenhanced magnetic resonance imaging (MRI) of the brain, which showed right high superior frontal gyrus parasagittal acute infarction as well as late acute infarctions of a tiny right anterior lower frontal cortical/subcortical white matter, right basal ganglia, right high posterior parietal parasagittal cortical area, and both right and left centrum semiovale. A further contrast-enhanced magnetic resonance angiography (MRA) of the brain showed left gangliocapsular small old infarct with gliotic changes. Head timeof-flight MRA (Fig. 2) showed significant diffuse narrowing of the visualized left ICA, left M1 segment of middle cerebral artery (MCA), and proximal posterior branch of left M2. Focal area of severe stenosis was also seen at the M1 segment of the right MCA. Lumber puncture was performed and the cerebrospinal fluid study was unremarkable in regard to protein level, glucose level, and white blood cell level. Autoimmune work up showed positive anti-nuclear antibody (1:640), positive anti-dsDNA antibodies (27.00), positive anti-thyroid peroxidase (169), and positive TSH receptor antibody. Positive antinuclear antibodies (ANA) and positive anti-dsDNA antibodies have been the subject of debate between the neurology and rheumatology teams. Finally, the rheumatology team ruled out systemic lupus erythematosus (SLE) as the case did not meet the criteria.
A conventional cerebral angiogram (Fig. 3) was highly suggestive of Moyamoya like disease with multiple vascular occlusions and numerous collaterals forming the “puff of smoke.” The patient was diagnosed with MMS secondary to GD, and she was started on carbimazole 20 mg twice daily, propranolol 40 mg twice daily, and intravenous hydrocortisone 100 mg 3 times daily. In addition, aspirin and statin were started. The neurosurgery team was consulted for possible extracranial-intracranial bypass surgery and after a discussion about the risks and benefits of the surgery with the patient, they decided to delay the surgery until thyroid function stabilizes. Hydrocortisone was discontinued after 3 days as the patient was vitally stable and the risk of thyroid storm was low, while the patient was maintained on aspirin, statin, carbimazole, and propranolol, and she was referred to a rehabilitation center for physical therapy.
At the rehabilitation center, the patient’s symptoms improved, and she partially regained the power in her left upper and lower extremities to perform most of her daily activities with minimal assistance. Follow-up thyroid function test showed TSH of <0.01 mIU/L, Free T3 of 4.3 pmol/L, and Free T4 of 19.7 pmol/L.

Figure 1: (a) Computed tomography (CT) head without contrast shows focal area of hypodensity in the right anterior frontal region likely representing recent infarction, along the right anterior carotid artery territory. (b) CT angiogram head with contrast shows severe smooth concentric attenuation of the left internal carotid artery (ICA) just distal to its origin with non-visualization of the supraclinoid segment and reconstitution of the left middle cerebral artery (MCA) through anterior communicating artery, and paucity of distal left MCA branches, mild concentric stenosis of almost entire left ICA is also noted just distal to the origin with tight stenosis at the supraclinoid and the terminal portion, hypoplastic and irregular left vertebral artery. Focal area of tight stenosis is seen at the origin of the right vertebral artery, focal area of severe stenosis seen at the M1 segment of the right MCA, hypoplastic left vertebral artery, terminated as left posterior inferior cerebellar artery, and double right posterior cerebral artery (PCA) with severe stenosis of the origin of fetal PCA
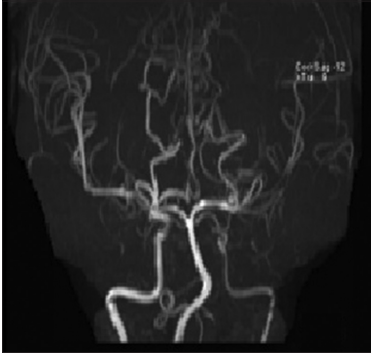
Figure 2: Head time-of-flight magnetic resonance angiography shows significant diffuse narrowing of the visualized left internal carotid artery, left M1 segment of middle cerebral artery (MCA). Focal area of severe stenosis is also seen at the M1 segment of the right MCA. There is a double right posterior cerebral artery (PCA) and hypoplastic left vertebral artery
The patient was scheduled for a follow-up with a neurosurgeon at 5 weeks to decide on extracranial-intracranial bypass surgery. However, she travelled to her country and did not show up.
DISCUSSION
MMS is a cerebrovascular disorder that occurs in association with other diseases, characterized by a slowly progressive steno-occlusive disease that first involves both distal ICAs and eventually progresses to involve both proximal ACAs and MCAs. Abnormal collateral vasculature develops in response to this process, mostly derived from the basal lenticulostriates and thalamoperforators [1,2,17].

Figure 3: Frontal and lateral projections of conventional angiogram of intracranial arteries show tight stenotic segment involving the distal right internal carotid artery and proximal A1 and M1 segments of the right anterior and middle cerebral arteries, respectively, with multiple tiny hairline collaterals forming the “puff of smoke” seen at the skull base
MMD is now observed worldwide with a bimodal age of onset, with children presenting around age 5 and adults presenting around age 40 [2]. The clinical presentations include transient ischemic attack, ischemic stroke, hemorrhagic stroke, seizure, headache, and cognitive dysfunction. MMS associated with GD mostly occurs in adult female patients. Their clinical symptoms are mainly ischemic lesions and usually occur during the state of hyperthyroidism [4,5,7-11]. The predominance of the female sex probably reflects the epidemiology of GD. Similarly, our case was an adult female who presented with ischemic stroke during the state of hyperthyroidism.
Although there are many case reports and few studies [4-16,11,18,19] have mentioned the relationship between MMS and GD, it is not clear whether this relationship is coincidental or causal, since the pathoetiological link has not yet been elucidated and, therefore, the mechanism of MMS and its relation to GD will remain a matter of debate. Despite this, various mechanisms have been postulated, suggesting genetic and autoimmune factors as important players in the pathogenesis of MMS in association with GD [20-22]. Interestingly, our patient has shown positive tests for antinuclear antibody and anti-dsDNA antibody, but did not meet the SLE criteria. ANAs have been frequently detected in patients with GD with uncertain clinical significance. Other autoantibodies associated with systemic autoimmune diseases such as anti-dsDNA antibodies and antibodies against the extractable nuclear antigens have been sporadically mentioned. In one study, anti-dsDNA antibodies were found in 82.0% of patients with GD compared to control [23]. It was found that anti-dsDNA antibodies increase the risk of cardiovascular disease in people with systemic lupus erythematosus by altering crucial biological processes, such as inflammation, the development of neutrophil extracellular traps, and apoptosis, which are responsible for a distinct and coordinated immune and vascular activation [24]. Similarly, we hypothesized that MMS in patients with GD is mediated through anti-dsDNA antibodies, by the same mechanisms. We reviewed the reported cases of MMS associated with GD in the literature; however, it is not clear how many cases showed circulating anti-dsDNA antibodies. Nevertheless, we believe that our case supports the role of autoimmunity in the pathogenesis of MMS in patients with GD. MMS is a cerebrovascular disorder that predisposes affected patients to infarction whenever the collateral vessels fail to compensate for the cerebral blood flow deficit produced by the progressive stenotic lesion in the major cerebral arteries [7]. Cerebrovascular ischemic symptoms in MMS associated with GD are usually precipitated by activities associated with thyrotoxicosis [9]. It was hypothesized that the hyperthyroid state caused an increase in cerebral metabolic demand and altered cerebral perfusion [9,20]. As a result, it is likely that the hyperthyroid state caused by GD in this patient increased the cerebral metabolic demand to the point where compensation from the collateral supply was insufficient, resulting in ischemic stroke. Once cerebral infarction or transient ischemic attacks develop in a patient with GD-related MMS, treatment with antithyroid therapy should be started immediately. Few reported cases of MMS with GD have shown symptomatic recovery with the improvement of arterial stenosis after antithyroid therapy [14]. Medical treatment with vasodilators, steroids, and antiplatelet agents has been tried with doubtful efficacy [9]. Cerebrovascular reconstruction surgery is an effective treatment for GD-related MMS. The keys to successful surgical treatment include timing the surgery during the euthyroid state and perioperative management, taking into account the thyroid function and the changes in cerebral hemodynamics [25]. Our patient presented with acute ischemic stroke and thyrotoxic state with a high Burch-Wartofsky score. Immediate initiation of carbimazole, propranolol, and hydrocortisone stabilized her condition with further improvement over the next 2 weeks. Her muscle power on the left side improved and she was able to manage most of her daily activities with minimal assistance until she returned to her country.
CONCLUSION
This is the first reported case of GD-related MMS in Qatar that presented with acute ischemic stroke. We hypothesized that MMS in our patient was mediated through anti-dsDNA antibodies, by various mechanisms that ultimately drive a distinctive and coordinated immune and vascular activation. Our patient became symptomatic because of thyrotoxicosis, and her neurological symptoms stabilized and ameliorated after antithyroid therapy. Therefore, patients with thyrotoxicosis secondary to GD who present with acute focal neurological symptoms should be evaluated for possible MMS, with prompt attempts to control the thyrotoxic condition. Further investigations are warranted to better understand the underlying mechanism with a focus on the role of anti-dsDNA antibodies in the pathogenesis of GD-related MMS.
AUTHORS’ CONTRIBUTION
Rafique S is the team leader who contributed to developing the work idea and composing and revising the manuscript.
Obaid KR contributed to writing the manuscript and reviewing the literature. Hassan IA preparing the images, contributing to writing the legends, and revising the manuscript. All authors read the manuscript and agree to its publication.
References
1. Demartini Z Jr., Teixeira BC, Koppe GL, et al. Moyamoya disease and syndrome: A review. Radiol Bras 2022;55:31-7.
2. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med 2009;360:1226-37.
3. Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ 2003;168:575-85.
4. Khandelwal P, Shah N, Prakash T, et al. Familial presentation of intracranial occlusive arteriopathy and ischemic stroke in patients with Graves’ hyperthyroidism. US Endocrinol 2015;11:89-91.
5. Kushima K, Satoh Y, Ban Y, et al. Graves’ thyrotoxicosis and moyamoya disease. Can J Neurol Sci 1991;18:140-2.
6. See TT, Lee SP, Tang CW. Moyamoya disease and thyrotoxic painless thyroiditis: A case report. J Intern Med Taiwan 2014;25:20-4.
7. Shen AL, Ryu SJ, Lin SK. Concurrent moyamoya disease and Graves’ thyrotoxicosis: Case report and literature review. Acta Neurol Taiwan 2006;15:114-9.
8. Ni J, Zhou LX, Wei YP, et al. Moyamoya syndrome associated with Graves’ disease: A case series study. Ann Transl Med 2014;2:77.
9. Cheon CK, Kim SY, Yoo JH. Two adolescent patients with coexistent Graves’ disease and Moyamoya disease in Korea. Korean J Pediatr 2014;57:287-91.
10. Nakamura H, Sato K, Yoshimura S, et al. Moyamoya disease associated with Graves’ disease and down syndrome: A case report and literature review. J Stroke Cerebrovasc Dis 2021;30:105414.
11. Li D, Yang W, Xian P, et al. Coexistence of moyamoya and Graves’ diseases: The clinical characteristics and treatment effects of 21 Chinese patients. Clin Neurol Neurosurg 2013;115:1647-52.
12. Suzuki S, Mitsuyama T, Horiba A, et al. Moyamoya disease complicated by Graves’ disease and Type 2 diabetes mellitus: Report of two cases. Clin Neurol Neurosurg 2011;113:325-9.
13. Malik S, Russman AN, Katramados AM, et al. Moyamoya syndrome associated with Graves’ disease: A case report and review of the literature. J Stroke Cerebrovasc Dis 2011;20:528-36.
14. Ishigami A, Toyoda K, Suzuki R, et al. Neurologic improvement without angiographic improvement after antithyroid therapy in a patient with Moyamoya syndrome. J Stroke Cerebrovasc Dis 2014;23:1256-8.
15. Ohba S, Nakagawa T, Murakami H. Concurrent Graves’ disease and intracranial arterial stenosis/occlusion: Special considerations regarding the state of thyroid function, etiology, and treatment. Neurosurg Rev 2011;34:297-304.
16. Ito H, Yokoi S, Yokoyama K, et al. Progressive stenosis and radiological findings of vasculitis over the entire internal carotid artery in moyamoya vasculopathy associated with Graves’ disease: A case report and review of the literature. BMC Neurol 2019;19:34.
17. Hsu SW, Chaloupka JC, Fattal D. Rapidly progressive fatal bihemispheric infarction secondary to Moyamoya syndrome in association with Graves thyrotoxicosis. AJNR Am J Neuroradiol 2006;27:643-7.
18. Li H, Zhang ZS, Dong ZN, et al. Increased thyroid function and elevated thyroid autoantibodies in pediatric patients with moyamoya disease: A casecontrol study. Stroke 2011;42:1138-9.
19. Kim SJ, Heo KG, Shin HY, et al. Association of thyroid autoantibodies with moyamoya-type cerebrovascular disease: A prospective study. Stroke 2010;41:173-6.
20. Liu JS, Juo SH, Chen WH, et al. A case of Graves’ diseases associated with intracranial moyamoya vessels and tubular stenosis of extracranial internal carotid arteries. J Formos Med Assoc 1994;93:806-9.
21. Garcin B, Louissaint T, Hosseini H, et al. Reversible chorea in association with Graves’ disease and moyamoya syndrome. Mov Disord 2008;23:620-2.
22. Tokimura H, Tajitsu K, Takashima H, et al. Familial moyamoya disease associated with Graves’ disease in a mother and daughter. Two case reports. Neurol Med Chir (Tokyo) 2010;50:668-74.
23. Pedro AB, Romaldini JH, Americo C, et al. Association of circulating antibodies against double-stranded and single-stranded DNA with thyroid autoantibodies in Graves’ disease and Hashimoto’s thyroiditis patients. Exp Clin Endocrinol Diabetes 2006;114:35-8.
24. Patiño-Trives AM, Pérez-Sánchez C, Pérez-Sánchez L, et al. Anti-dsDNA antibodies increase the cardiovascular risk in systemic lupus erythematosus promoting a distinctive immune and vascular activation. Arterioscler Thromb Vasc Biol 2021;41:2417-30.
25. Endo H, Fujimura M, Niizuma K, et al. Efficacy of revascularization surgery for moyamoya syndrome associated with Graves’ disease. Neurol Med Chir (Tokyo) 2010;50:977-83.
