Full HTML
Immunoglobulin G4-related disease: A narrative review
Abdo Qaid Lutf, Salah Mahdy
Author Affiliation
1Consultant
2Srenior Consultant, Rheumatology Division, Department of Medicine, Alkhor Hospital, Alkhor, Qatar
Abstract
Immunoglobulin G4-related disease (IgG4-RD) is a rare and new but increasingly recognized immune-mediated fibroinflammatory condition known to affect multiple organs. The diagnostic approach is challenging, as there is no single investigation to confirm the diagnosis, which requires the integration of clinical, biochemical, and radiographic manifestations with classic histopathologic features to establish the diagnosis. The histology of IgG4-RD is determined by a dense lymphoplasmacytic infiltrate, storiform fibrosis, and obliterative phlebitis in the presence of an increased level of IgG4 in most patients. The first line of treatment is systemic glucocorticoids, but adverse effects of the drug, suboptimal response, and disease recurrences on reduction or termination of therapy highlight the need for an alternative therapy such as rituximab, which appears to be a promising alternate agent in the treatment of IgG4-RD; however, its efficacy needs to be evaluated in large clinically controlled trials.
DOI: 10.32677/yjm.v1i2.3533
Keywords: Autoimmune, Immunoglobulin G4-related disease, Lymphoplasmacytic infiltration, Storiform fibrosis
Pages: 65-68
View: 7
Download: 9
DOI URL: https://doi.org/10.32677/yjm.v1i2.3533
Publish Date: 28-03-2025
Full Text
Immunoglobulin G4-related disease (IgG4-RD) is a new and evolving immune-mediated disease characterized by focal or diffuse organ infiltration by immunoglobulin G4-bearing plasma cells which, if left untreated, leads to irreversible fibrosis, organ dysfunction, and death [1,2]. Various terms were used to describe this clinical phenomenon until 2011 when the term IgG4-RD was first proposed and later approved in 2012 by an international multidisciplinary study group [3,4]. Pancreatobiliary tract, retroperitoneum/aorta, head and neck, and salivary glands are the most commonly observed disease phenotypes [3], but IgG4-RD can affect any organ in the body with a variety of clinical features that mimic malignant, infectious, and inflammatory conditions, making diagnosis difficult. Therefore, a high index of suspicion is necessary for the early detection of this disorder, to avoid irreversible damage and death. The diagnostic work-up should include clinical, biochemical, and radiological findings with classic histopathological features essential for diagnosis [1,4,5]. Given the absence of a single test for IgG4-RD, criteria are suggested to establish the diagnosis of this clinical entity. The current recommendation for the first-line treatment of IgG4-RD is systemic corticosteroids, while rituximab appears to be a promising alternative agent when steroids are intolerable or have a suboptimal response. In this review, we summarize the latest advances in the diagnosis and treatment of IgG4-RD, focusing on the current diagnostic approaches therefor.
EPIDEMIOLOGY
The exact worldwide incidence of this disease is not known, as it is a relatively new entity and largely underestimated in clinical practice, and most available data come from sporadic reports, particularly from some Asian countries like Japan [6,7]. While IgG4-RD is described in nearly all racial and ethnic groups, around 75% of the reported cases are from Japan, with an incidence of 0.28–1.08/100,000 population [7]. With a few exceptions, IgG4-RD typically affects middleaged people between 50 and 70 and predominantly Asian males, as the studies have shown a male/female ratio varying between1.6:1 and 4:1 [6,7]. The reason for the male sex predominance is unclear.
ETIOPATHOGENESIS
The etiopathogenesis of IgG4-RD is not yet fully understood. The current evidence suggests that IgG4-RD is an antigen-driven immune-mediated process, with important roles for both B and T cells, especially CD4+ and T-follicular helper cells (Tfh); however, the nature of the antigen(s) and the reason for the disease targeting particular organs remain unclear. Recently, several candidate autoantigens have been implicated such as galectin-3, laminin 111, and annexin A11, which suggests that there is more than 1 autoantigen that can trigger this condition [8-10]. T cells are commonly linked to disease pathogenesis due to the observation that many CD4+ T cells are present at the sites of inflammation in IgG4-RD. Recent studies have demonstrated that an unconventional population of CD4+ cytotoxic T lymphocytes that express signaling lymphocytic activation module (SLAM) F7+ is central to the pathogenesis of the disease, and these cells are sustained by continuous antigen presentation by cells of the B lymphocyte lineage, including but not limited to plasmablasts [11].
CLINICAL PRESENTATION
While the clinical presentation of IgG4-RD varies widely depending on the organ affected, it is usually subacute or chronic and discovered incidentally by clinical examination (thyroid enlargement, salivary or lacrimal gland swelling, or lymphadenopathy), through radiological findings, or unexpectedly in pathological specimens [1,5,12]. Constitutional symptoms such as fever, malaise, and night sweats are unusual [13]. The symptoms most commonly associated with tumefaction may occur as localized masses or nodules such as in orbit, kidney, and lung or as diffuse enlargement of an organ such as the pancreas [5,12]. Some patients may experience severe complications of obstruction or compression, including obstructive jaundice in autoimmune pancreatitis or IgG4 sclerosing cholangitis, visual disturbance in IgG4-related dacryoadenitis, and hydronephrosis in IgG4-related retroperitoneal fibrosis [1,5,12]. IgG4-RD can involve one or multiple organs and can affect any organ except synovial tissue [7]. Multiple organs are affected in 60–90% of patients with IgG4-RD and symptoms of asthma or allergy are present in approximately 40% of the patients [14].
DIAGNOSIS The diagnosis of IgG4-RD remains an important clinical challenge, as the clinical picture is variable and, in many cases, can mimic a malignant disease. Therefore, early diagnosis of IgG4-RD is crucial to prevent unwanted surgical resection. There is no simple diagnostic test that clearly indicates the presence of IgG4-RD. Instead, the collection of clinical, radiological, pathological, and laboratory modalities, including serology, is required to suggest the diagnosis. In general, the diagnosis should be suspected in cases of idiopathic pancreatitis, sclerosing cholangitis, salivary/lacrimal gland enlargement, retroperitoneal fibrosis, and orbital pseudotumor/proptosis, followed by a serum IgG4 study. Although it is not diagnostic, an elevated serum IgG4 level supports the diagnosis because it is present in only 20–30% of the patients and does not correlate with disease activity [15]. Carruthers et al. estimated that the sensitivity and the negative predictive values of elevated serum IgG4 level (>180 mg/dL) are 90% and 96%, respectively, with low specificity and positive predictive values [16].
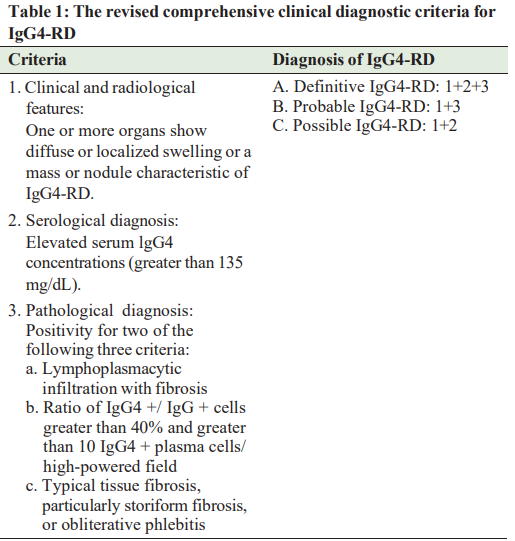
predictive values [16]. The main problem in the diagnosis of IgG4-RD is that it frequently presents with clinical and radiological findings that mimic malignancy. Therefore, regardless of the organ involvement, histological confirmation of the disease is crucial to differentiate IgG4 RD from malignant tumors to avoid misdiagnosis and initiate treatment as soon as possible. The three major histopathological features associated with IgG4-RD are dense lymphoplasmacytic infiltrate with a high level of IgG4+ plasma cell, storiform fibrosis, and obliterative phlebitis [15,17-19]. Just like IgG4 levels, clinical, laboratory, imaging, and histopathological findings too are never totally exclusive for IgG4-RD, which makes valid diagnostic criteria hard to establish. As there is no single diagnostic test available for diagnosing IgG4-RD, several diagnostic criteria have been proposed to establish the diagnosis. In 2011, Umehara et al. proposed the original comprehensive clinical diagnostic (CCD) criteria for IgG4-RD [3], and in 2020, the revised version of CCD [20] was released (Table 1). Since then, several organ-specific criteria such as IgG4-related autoimmune pancreatitis and IgG4-related kidney disease have been suggested [21,22]. When patients cannot be diagnosed using the CCD criteria, organ-specific diagnostic criteria for IgG4-RD may be used. Classification criteria for IgG4-RD have been developed and validated in 2019 by the American College of Rheumatology/ European League against Rheumatism (ACR/EULAR) [23]. The criteria were developed primarily to identify patients for inclusion in clinical trials and other studies and are not intended for diagnostic use in clinical practice. The approach to these criteria begins with step 1, the so-called entry criteria, in which the patient should demonstrate a characteristic clinical or radiological involvement of a typical organ and pathological evidence of an inflammatory process accompanied by a lymphoplasmacytic infiltrate of unclear etiology in one of these organs. Step 2 is called exclusion criteria, which comprises 32 clinical, serological, radiological, and pathological items. The presence of any of these items eliminates the patient from IgG4-RD classification. Step 3 comprises the inclusion criteria, namely, the application of eight weighted inclusion criteria domains that address clinical findings, serological results, radiological assessments, and pathological interpretations. Within each criterion domain, items were arranged according to the degree to which they either increased or decreased the likelihood of classification. The IgG4-RD classification criteria are fulfilled if entry criteria are met, no exclusion criteria are present, and the total inclusion criteria point total ≥20 [23].
MANAGEMENT AND OUTCOMES
Once the diagnosis of IgG4-RD is confirmed, treatment with steroids is the recommended first-line therapy, unless there is a major contraindication for steroid use [5,19]. Response to steroid therapy in the form of clinical improvement, decrease in the size of the enlarged organs and masses with improvement in organ function, and usually, a decrease in serum levels of IgG4, is seen within days or weeks, and remission can be achieved within months in the majority of patients. Remission induction is commonly initialized with 30–40 mg/day of prednisone or 0.6 mg/kg of body weight per day for 2–4 weeks, followed by a slow tapering of steroids over 3–6 months [19,24,25]. Eventual relapse frequently occurs, despite the initial response to systemic glucocorticoid therapy. Elevated IgG4 serum concentration, as well as extensive organ involvement, has been linked to an increased likelihood of relapse [26,27]. Recurrence (relapse) of the disease during steroid tapering or following the completion of steroid therapy has been documented in 40% of patients with IgG4-RD [5], raising the ongoing debate of whether long-term maintenance therapy is required for all patients with IgG4-RD [5,12,19]. Relapse is treated with a repetition of the steroid course with slower tapering or with steroid-sparing (e.g., disease-modifying antirheumatic drugs [DMARDs] and rituximab) agents, preferably rituximab which has shown promising results in patients who are refractory to steroids [28]. Evidence-based reports on the efficacy of steroid-sparing agents either singly or in combination with steroids in the treatment of IgG4-RD are scant, with conflicting results. A meta-analysis of 15 studies of 15 observational, uncontrolled, non-randomized clinical trials involving 1169 patients showed that using a combination of DMARDs such as azathioprine, mycophenolate mofetil, and methotrexate with steroids is associated with a higher remission rate with an odds ratio of OR = 3.36, 95% CI [1.44–7.83] [29]. However, a retrospective study comparing immunosuppressants with steroid monotherapy in the treatment of AIP relapses found no significant difference in relapse-free survival between the treatments [26]. On the other hand, the introduction of B-cell depletion with rituximab has shown promising preliminary results, as it leads to a decrease in IgG4-positive plasma cells and serum IgG4, together with decreased tissue fibrosis. However, its efficacy needs to be evaluated in large clinically controlled trials [5,12,18,26]. Responsiveness to medical therapy is limited in late-stage disease and the case of extensive fibrosis, although case reports have shown that the efficacy of steroids for IgG4-related retroperitoneal fibrosis is the same as for IgG4-RD in other organs [30]. Surgery may also be considered in cases where symptoms are related to a mass effect from a pseudotumor [31].
DISEASE ACTIVITY FOLLOW-UP
Since disease biomarkers might be normal at illness onset and are, therefore, useless for determining treatment response and relapse prediction, none of the currently available disease biomarkers alone can generally be considered a trustworthy mirror of disease activity [32]. However, the following actions were recommended to monitor disease activity: Clinical evaluation of all possibly affected organs; peripheral eosinophil count monitoring; measurement of total serum levels of IgE, IgG, and IgG4; and inflammatory markers, and tracking changes seen in the results of images of affected organs [33].
CONCLUSION
IgG-RD is a chronic inflammatory disease that often runs an indolent course and may affect virtually any organ of the body. It could be misdiagnosed clinically as malignancy. Diagnosis remains a challenge. However, the revised CCD criteria for IgG4-RD and the recent EULAR diagnostic criteria are very comprehensive and serve as a useful guide. The first line of treatment is systemic glucocorticoids, but adverse effects of the drug and disease recurrences on reduction or termination of therapy highlight the need for favorable therapy. Rituximab appears to be a promising agent in the treatment of IgG4-RD if there is intolerance or suboptimal response to steroids, but its efficacy needs to be evaluated in large clinically controlled trials. Surgery may also be considered in cases where symptoms are related to a mass effect from a pseudotumor.
AUTHORS’ CONTROBUTIONS
Lutf AQ aided in conceptualization, methodology, literature review, manuscript preparation, critical review, and revisions of the manuscript. Mahdy S aided in the literature review, manuscript writing, critical review, and revisions of the manuscript. Both authors reviewed and approved the final version of the manuscript
References
1. Obiorah IE, Velasquez AH, Özdemirli M. The clinicopathologic spectrum of IgG4-related disease. Balkan Med J 2018;35:292-300.
2. Okazaki K, Umehara H. Current concept of IgG4-related disease. Curr Top Microbiol Immunol 2017;401:1-17.
3. Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012;2291:21-30.
4. Maritati F. Peyronel F, Vaglio A. IgG4-related disease: A clinical perspective Rheumatology 2020;59:iii123-31.
5. Hegade VS, Sheridan MB, Huggett MT. Diagnosis and management of IgG4-related disease. Frontline Gastroenterol 2019;10:275-83.
6. Karadeniz H, Vaglio A. IgG4-related disease: A contemporary review. Turk J Med Sci 2020;50(SI-2):1616-31.
7. Uchida K, Masamune A, Shimosegawa T, et al. Prevalence of IgG4-related disease in Japan based on nationwide survey in 2009. Int J Rheumatol 2012;2012:358371.
9. Shiokawa M, Kodama Y, Sekiguchi K, et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med 2018;10:eaaq0997.
10. Hubers LM, Vos H, Schuurman AR, et al. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut 2018;67:728.
11. Mattoo H, Mahajan VS, Maehara T, et al. Clonal expansion of CD4(+) cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol 2016;138:825.
12. Lang D, Zwerina J, Pieringer H. IgG4-related disease: Current challenges and future prospects. Ther Clin Risk Manag 2016;12:189-99.
13. Yamamoto M, Takahashi H, Shinomura Y. Mechanisms and assessment of IgG4-related disease: Lessons for the rheumatologist. Nat Rev Rheumatol 2013;10:148-59.
14. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Eng J Med 2012;366:539-51.
15. Ikeda T, Oka M, Shimizu H, et al. IgG4-related skin manifestations in patients with IgG4-related disease. Eur J Dermatol 2013;23:241-5.
16. Carruthers MN, Khosroshahi A, Augustin T, et al. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015;74:14-8.
17. Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: Dataset of 235 consecutive patients. Medicine (Baltimore) 2015;94:e680.
18. Campochiaro C, Ramirez GA, Bozzolo EP, et al. IgG4-related disease in Italy: Clinical features and outcomes of a large cohort of patients. Scand J Rheumatol 2016;45:135-45.
19. Zhang W, Stone JH. Management of IgG4-related disease. Lancet Rheum 2019;1:e55-65.
20. Umehara H, Okazaki K, Kawa S, et al. Research program for intractable disease by the ministry of health, labor and welfare (MHLW) Japan. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol 2021;31:529-33.
21. Shimosegawa T, Chari ST, Frulloni L, et al. International association of pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the international association of pancreatology. Pancreas 2011;40:352-8.
22. Kawano M, Saeki T, Nakashima H, et al. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol 2011;15:615-26.
23. Wallace ZS, Naden RP, Chari S, et al. The 2019 American college of8. Perugino CA, AlSalem SB, Mattoo H, et al. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. J Allergy Clin Immunol 2019;143:736. rheumatology/European league against rheumatism classification criteria for IgG4-related disease. Arthritis Rheumatol 2020;72:7-19.
24. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol 2015;67:1688-99.
25. Kamisawa T, Okazaki K, Kawa S, et al. Amendment of the Japanese consensus guidelines for autoimmune pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J Gastroenterol 2014;49:961-70.
26. Hart PA, Topazian MD, Witzig TE, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: The mayo clinic experience. Gut 2013;62:1607-15.
27. Grewal K, Cohen P, Kwon JS, Kaufman DA. IgG4-related disease presenting as a lung mass and weight loss: Case report and review of the literature. Respir Med Case Rep 2016:17:27-9.
28. Campochiaro C, Della-Torre E, Lanzillotta M, et al. Long-term efficacy of maintenance therapy with Rituximab for IgG4-related disease. Eur J Intern Med 2020;74:92-8.
29. Omar D, Chen Y, Cong Y, et al. Glucocorticoids and steroid sparing medications monotherapies or in combination for IgG4-RD: A systematic review and network meta-analysis. Rheumatology (Oxford) 2020;59:718-26.
30. Zen Y, Onodera M, Inoue D, et al. Retroperitoneal fibrosis a clinicopathologic study with respect to immunoglobulin G4. Am J Surg Pathol 2009;33:1833-9.
31. Osuorji C, Master K, Osuorji I. IgG4-related disease with renal and pulmonary involvement. Cureus 2021;13:e17071.
32. Lanzillotta M, Mancuso G, Della-Torre E. Advances in the diagnosis and management of IgG4 related disease. BMJ 2020;369:m1067.
33. Chen Y, Cai S, Dong L, et al. Update on classification, diagnosis, and management of immunoglobulin G4-related disease. Chin Med J 2022;135:381-92.
