Full HTML
Frequency of statin-induced liver injury: A secondary analysis of previous hospital-based study
Abdel-Monem Badawi Yousif, Ebtihal Abdelmoneim Hassan
Author Affiliation
1 Senior Pharmacist, Department of Pharmacy,
2Consultant, Department of Accident and Emergency, Hamad General Hospital, Doha, Qatar
Abstract
Background:Although statins are considered safe, they do have side effects with a wide range of hepatic adverse effects. The present study aims to estimate the frequency of liver injury in patients treated with various statins and to describe their clinical characteristics and outcomes. Materials and Methods: We carried out a secondary post hoc analysis of collected data from our previous study entitled “Frequency of Rhabdomyolysis in Patients Treated with Statins in Hamad General Hospital, Qatar.” Results: We identified 10 cases (1.0%) of statin-induced liver injury during the study period. Their mean age was 62±10.09 years, with 6 (60%) males and 4 (40%) females. Of the 10 patients, six patients received rosuvastatin, two patients received atorvastatin, and other two cases received simvastatin. The mean duration between the initiation of statin and the development of liver injury (latency period) was 20.40±6.91 months. Five of our patients were asymptomatic, and liver injury was discovered incidentally during routine testing of the patients during routine follow-up, while four patients developed painless jaundice and one developed muscle pain attributed to rhabdomyolysis. Statins were stopped in all patients. Nine of them were managed on an outpatient basis, while one patient with rhabdomyolysis was admitted. In all patients, other statins were reintroduced after a mean time of 7.4±3 months without recurrence of liver injury. No mortality has been reported. Conclusion: Our study demonstrated that statin-induced liver injury is a rare clinical entity that occurs regardless of the dose and type of statin, with rosuvastatin being the most causative drug. Statin-induced liver injury was asymptomatic and was discovered incidentally in 50% of our cases during routine testing, underscoring the importance of routine follow-up of liver function tests in asymptomatic patients.
DOI: 10.32677/yjm.v1i1.3286
Keywords: Atorvastatin, Rosuvastatin, Simvastatin, Statin-induced liver injury
Pages: 27-30
View: 4
Download: 8
DOI URL: https://doi.org/10.32677/yjm.v1i1.3286
Publish Date: 27-03-2025
Full Text
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are among the most prescribed drugs worldwide, which have seen rapid growth in recent years given encouraging data from clinical trials on the utility of these agents in the primary and secondary prevention of cardiovascular disease. More than 143 million prescriptions are issued annually in the United States of America [1]. Although statins are considered safe, they do have side effects with a wide range of hepatic adverse effects. Asymptomatic and usually temporary elevation of transaminases is among the most common side effects, and it is rarely clinically significant and usually occurs in the early stages of therapy [2]. This phenomenon is called transaminitis as it is thought to be related to alteration of hepatocyte cellular membrane with enzyme leakage rather than direct liver injury [3]. In a systematic review conducted by Kashani et al., the absolute risk of transaminase elevations was significantly higher with statin therapy in comparison with placebo [4]. Nevertheless, significant elevations of these enzymes are rare and have been reported in 1–3% of cases [5-7]. Statins are among the most frequently prescribed drugs in Qatar, with more than 120,000 prescriptions issued each year. However, little is known about the side effects of statins, especially liver damage. The present study aims to estimate the frequency of liver injury in patients treated with various statins and to describe their clinical characteristics and outcomes in our local setting.
MATERIALS AND METHODS Study
Design, Setting, and Population
We carried out a secondary post hoc analysis of collected data from our previous study entitled “Frequency of Rhabdomyolysis in Patients Treated with Statins in Hamad General Hospital, Qatar” [8] with a modified objective to estimate the frequency of statin-associated liver injury in this cohort. The primary study was designed to assess the frequency of rhabdomyolysis in patients treated with different statins for the period between January 1, 2017 and December 31, 2017, and it was approved by the Medical Research Committee at Hamad Medical Corporation (# MRC-01-18-232).
Definitions, Inclusion, and Exclusion Criteria
The liver injury was defined as follows [6]:
1. Raising alanine transaminase (ALT) or aspartate transaminase levels ≥5 upper level of normal (ULN)
2. Alkaline phosphatase ≥2 ULN
3. The combination of an increase in ALT >3 ULN and total bilirubin >2 ULN
Cases that met the criteria for liver injury, in addition to the chronology between statin drug use and the onset of hepatic dysfunction with subsequent rapid improvement on cessation of statins, were included in the study, while patients with other causes of liver injury were excluded from the study.
Data Analysis
Data were reported as the mean±standard deviation (SD) with the range for quantitative variables, whereas qualitative variables were described as numbers and percentages.
RESULTS
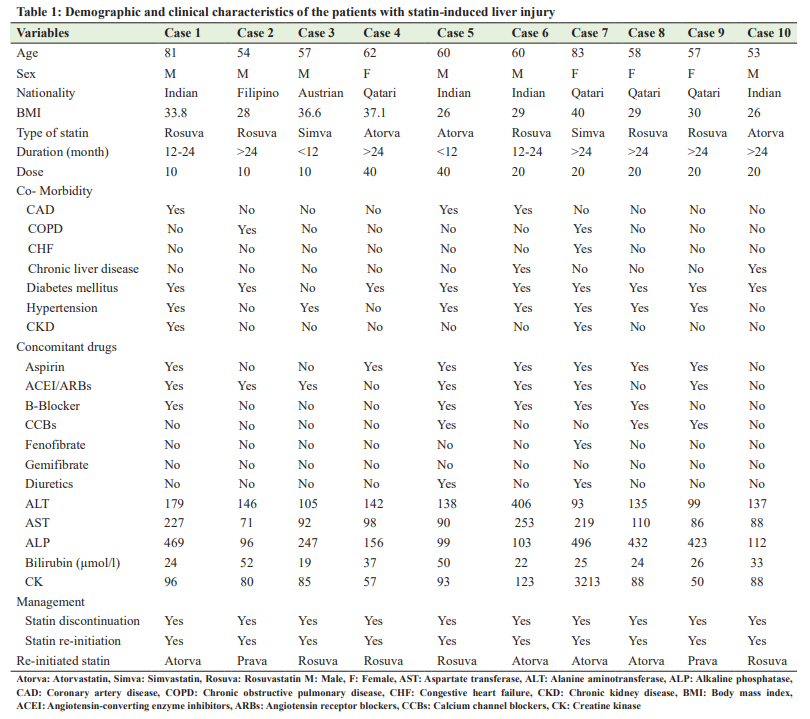
Among the 1000 patients recruited in the primary study, we identified only 10 cases (1.0%) with statin-induced liver injury during the study period. Their mean age was 62±10.09 years (range: 53–83 years), with 6 (60%) males and 4 (40%) females. Of the 10 patients with liver injury, six patients received rosuvastatin, two patients received atorvastatin, and other two cases received simvastatin. The mean duration between the initiation of statin and development of liver injury (latency period) was 20.40±6.91 months (range: 8–27 months). Five of our patients were asymptomatic and liver injury was discovered incidentally during routine testing of the patients during routine follow-up, while four patients developed painless jaundice and one developed muscle pain attributed to rhabdomyolysis. Statins were stopped in all patients. Nine of them were managed on an outpatient basis, while one patient with rhabdomyolysis was admitted and received intravenous fluid. In all patients, other statins were reintroduced after a mean time of 7.4±3 months (range: 2–12 months) without recurrence of liver injury. Table 1 presents the demographic and clinical characteristics of patients who developed statin-induced liver injury. No mortality has been reported.
DISCUSSION
There is a paucity of studies on the frequency of statin-induced liver injury, with most data drawn from case reports or small retrospective or prospective studies. To the best of our knowledge, our study is the first in Qatar to describe the incidence of statininduced liver injury. Initially, statins were thought to have a high risk of causing drug-induced liver injury. When first approved for use in the United States, baseline liver function tests (LFTs) were recommended before starting the statin and routine monthly monitoring thereafter [1]. Since then, the significance and utility of routinely monitoring LFTs following the initiation of statin therapy have been a subject of debate until NICE guidelines recommended baseline LFTs testing before initiating statin therapy, then within 3 months, and at 12 months, followed by testing only if the patient develops any symptoms related to the side effects of statins [9]. On the other hand, the American College of Cardiovascular Administrators/American Hospital Association guidelines recommend measuring baseline LFTs, followed by testing only when clinically indicated as prior efficacy trials have demonstrated no benefit in preventing clinically significant liver injury [1,10]. However, our findings, showing that 50% of our cases were discovered incidentally during routine testing, highlight the challenging question of monitoring for liver injury in patients receiving statin therapy. Thus, we recommend the routine follow-up testing of LFTs in asymptomatic patients at least in the initial few months when the risk of hepatotoxicity is highest. The occurrence of statin-induced liver injury has been reported by some studies with different frequencies, based on the criteria utilized for diagnosis. The incidence of true liver injury caused by statin is noticed to be around 1% [11], which is consistent with our finding. A characteristic element of statin-induced liver injury is that it may present with several phenotypes capable of resembling other liver diseases. Moreover, since specific markers for hepatotoxicity are still not available, it is difficult to prove causality without rechallenge, which is not without risk, leading some researchers to recommend against rechallenge if significant liver injury occurs [12]. However, in the study by Bjornsson et al. [13], statin-induced liver damage could only be confirmed in three out of 73 cases with rechallenge, and the diagnosis depended mostly on the exclusion of other causes as well as a consistent chronology between drug intake and the onset of liver dysfunction. Similarly, in our study, rechallenge was not applied, and the diagnosis was based on the exclusion of other causes as well as a consistent timeline between drug ingestion and the onset of liver impairment and return of transaminases to baseline. Idiosyncratic liver injury due to statins has been reported in 1.9–5.5% of patients in a prospective series of drug-induced liver injury [14]. Rather, we found that liver damage occurred regardless of statin dosage in all patients. The interval between drug initiation and the development of liver injury (latency period) varies between 5 weeks and 6 months [1,3,5,6,10-13]. However, prolonged latency periods for a year or more have been reported [1,15]. In our series, the mean latency period was 20.40±6.91 months (range: 8–27 months), and 80% of the cases exhibited prolonged latency periods for a year or more. Atorvastatin is the most common cause of clinically significant liver injury among statins with a reported incidence of 1/17 000 users. On the contrary, our study demonstrated that rosuvastatin was the drug most implicated in liver injury. The mechanism of statin-induced liver damage remains unclear and various mechanisms of hepatotoxicity have been proposed including mitochondrial dysfunction [16], immunemediated [17], and the inhibition of the respiratory chain [18]. There are limited data on the risk of cross-toxicity between different statins in cases of statin-induced liver injury, and some authors discourage restarting statin after an episode of liver toxicity unless clinically necessary and no other suitable alternative is available. However, Liu et al. [19] reported two cases of atorvastatin-induced liver injury who were switched to pravastatin following the return of transaminases to baseline. Similarly, all patients in this series were switched to different statins following the return of transaminases to baseline without recurrence of liver injury (Table 1). Patients with statin-induced liver injury generally have a good prognosis, with few reported fatalities associated with atorvastatin and simvastatin [14,15]. In our series, the outcomes were favorable for all types of statins and no mortality has been reported. Several limitations to the present study should be acknowledged. These limitations included the retrospective design, the small sample size (attributed to the low incidence of statin-induced liver injury) which did not allow us to perform advanced analysis to determine risk factors for statin-induced liver injury, and finally the implementation at a single center, making it difficult to generalize the study results.
CONCLUSION
Our study demonstrated that statin-induced liver injury is a rare clinical entity that occurs regardless of the dose and type of statin, with rosuvastatin being the most causative drug. Statin-induced liver injury was asymptomatic and was discovered incidentally in 50% of our cases during routine testing, underscoring the importance of routine follow-up of LFTs in asymptomatic patients. Statin withdrawal is the best therapeutic option, once liver injury is identified or suspected with switching to different statins following the return of transaminases to baseline.
References
1. Russo MW, Hoofnagle JH, Gu J, et al. Spectrum of statin hepatotoxicity: Experience of the drug-induced liver injury network. Hepatology 2014;60:679-86.
2. Clarke AT, Mills PR. Atorvastatin associated liver disease. Dig Liver Dis 2006;38:772-7.
3. Jose J. Statins and its hepatic effects: Newer data, implications, and changing recommendations. J Pharm Bioallied Sci 2016;8:23-8.
4. Chalasani N. Statins and hepatotoxicity: Focus on patients with fatty liver. Hepatology 2005;41:690-5.
5. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: A systematic overview of randomized clinical trials. Circulation 2006;114:2788-97.
6. Chitturi S, George J. Hepatotoxicity of commonly used drugs: Nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis 2002;22:169-83.
7. Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011;55:683-91.
8. Perdices EV, Medina-Cáliz I, Hernando S, et al. Hepatotoxicity associated with statin use: Analysis of the cases included in the Spanish hepatotoxicity registry. Rev Esp Enferm Dig 2014;106:246-54.
9. Yousif AB, Hassan EA, Ahmed MU, et al. Frequency of rhabdomyolysis in patients treated with statins in hamad general hospital, Qatar. Libyan J Med Sci 2021;5:75-8.
10. National Institute for Health and Care Excellence. Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification; 2016. Available from: https://www.nice.org.uk/guidance/cg181 [Last accessed on 2022 Jan 01].
11. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 2014;129:1-45.
12. Chang CY, Schiano TD. Review article: Drug hepatotoxicity. Aliment Pharmacol Ther 2007;25:1135-51.
13. Cohen DE, Anania FA, Chalasani N, et al. An assessment of statin safety by hepatologists. Am J Cardiol 2006;97:77-81.
14. Bjornsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: Reports of idiosyncratic liver injury post-marketing. J Hepatol 2012;56:374-80.
15. Björnsson ES. Hepatotoxicity of statins and other lipid-lowering agents. Liver Int 2017;37:173-8.
16. Tolosa L, Carmona A, Castell JV, et al. High-content screening of druginduced mitochondrial impairment in hepatic cells: Effects of statins. Arch Toxicol 2015;89:1847-60.
17. Pelli N, Setti M, Ceppa P, et al. Autoimmune hepatitis revealed by atorvastatin. Eur J Gastroenterol Hepatol 2003;15:921-4.
18. Halegoua-De Marzio VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In: Kaplowitz N, DeLeve L, editor. Drug-Induced Liver Disease. 3rd ed. United States: Academic Press; 2013. p. 519-40.
19. Liu Y, Cheng Z, Ding L, et al. Atorvastatin-induced acute elevation of hepatic enzymes and the absence of cross-toxicity of pravastatin. Int J Clin Pharmacol Ther 2010;48:798-802.
