Full HTML
Is glucose-6-phosphate dehydrogenase deficiency a risk factor for melioidosis?
Almurtada Razok1 , Zohaib Yousaf2 , Abdo Lutf3 , Musaed Alsamawi4
Author Affiliation
1Resident,
2Consultant, Department of Medicine, Hamad General Hospital, Doha, Qatar,
3Consultant, Rheumatology Division, 4Consultant, Infectious Diseases Division, Department of Medicine, Alkhor hospital, Qatar
Abstract
Melioidosis is an infection that causes high morbidity and mortality. Predisposing risk factors include diabetes mellitus (DM) and immunocompromised state. We report a case of septic shock secondary to bacteremia caused by Burkholderia pseudomallei in a patient who is supposed to be previously healthy and subsequently diagnosed with DM and glucose-6-phosphate dehydrogenase (G6PD) deficiency. He completed 17 days of intravenous antibiotics followed by a 12-week course of oral trimethoprim/sulfamethoxazole. We postulate that G6PD deficiency could be a risk factor for melioidosis.
DOI: 10.32677/yjm.v1i1.3291
Keywords: Burkholderia pseudomallei, Glucose-6-phosphate dehydrogenase deficiency, Hemolysis, Melioidosis
Pages: 49-51
View: 6
Download: 7
DOI URL: https://doi.org/10.32677/yjm.v1i1.3291
Publish Date: 27-03-2025
Full Text
Melioidosis is caused by the Gram-negative bacterium Burkholderia pseudomallei. It is a life-threatening infection endemic in Southeast Asia and North Australia with high case-fatality rates in animals and humans [1]. In the rest of the world, the current information on the epidemiology of melioidosis is very scanty and it is derived mostly from case reports and series. However, melioidosis appears not to be rare in Bangladesh but underreported or as-yetunrecognized foci of infection may exist, due to lack of awareness among microbiologists and clinicians and lack of diagnostic microbiology infrastructure [2]. In Qatar, the disease is rare among Qatari residents and sporadic cases of melioidosis have been reported in non-Qatari patients, especially South Asians, with diabetes mellitus (DM) being the main risk factor [3,4]. In the present work, we are reporting a case of melioidosis in a Bangladeshi patient with newly diagnosed DM and glucose-6-phosphate dehydrogenase (G6PD) deficiency.
CASE REPORT
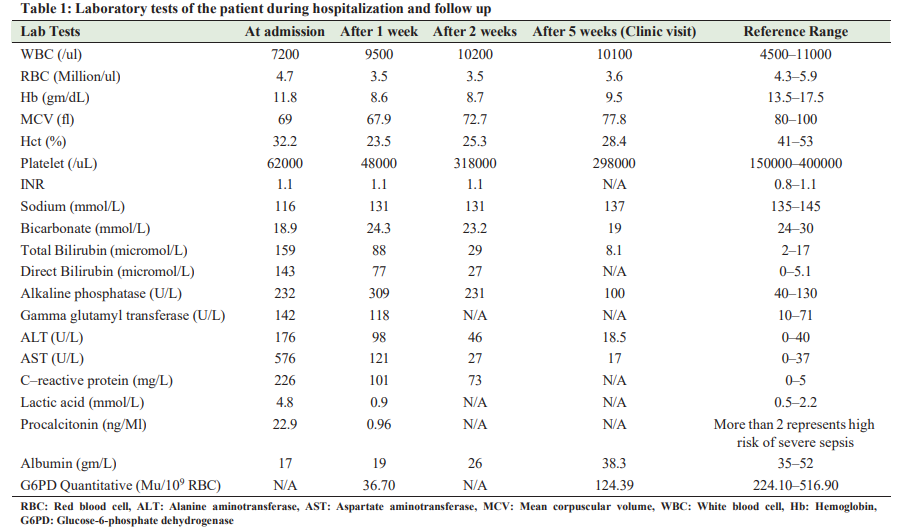
A 40-year-old Bangladeshi gentleman, previously fit and healthy, presented with fever that lasted for 10 days. He was a farmer and was directly exposed to farm animals. He was an active smoker with smoking history of 15 packs a year. He was recently on a trip to India, 2 weeks before his presentation, with no identifiable sick contacts. Two days later, he developed dry cough, myalgia, chills, decreased appetite, and passing dark urine. No hemoptysis,weight loss, chest pain, or vomiting were observed. Clinical examination on presentation revealed that the patient was sick but conscious (Glasgow Coma Scale [GCS] of 15/15) with jaundice and a temperature of 39.5°C, a pulse rate of 112 beats/min, and normal blood pressure. The rest of the physical examination was unremarkable. Intravenous paracetamol was administered and blood samples were collected and sent to the laboratory for cultures. Four hours later, the patient became drowsy with a fall in GCS to 12/15. Moreover, his blood pressure fell to 80/60 mmHg with no further interval changes on his physical examination. He received 2 g of ceftriaxone empirically and fluids intravenously. Despite resuscitation with an additional fluid bolus of 2 l, his blood pressure did not improve. The patient was diagnosed with septic shock and ceftriaxone was escalated to piperacillin/tazobactam. He was transferred to the medical intensive care unit, where he received noradrenaline infusion and a bolus of hydrocortisone through central venous line. Chest X-ray was conducted, revealing a consolidation in the left mid and lower zones with atelectatic changes along the right lung base. The preliminary blood culture report indicated Gram-negative bacilli and the infectious disease team initiated meropenem while awaiting the identification of the microorganism and the sensitivity report. On day 3, the noradrenaline infusion was discontinued and the final blood culture report identified B. pseudomallei. Based on sensitivity, meropenem was deescalated to ceftazidime. However, the fever continued till day 8 of hospitalization. Repeat cultures were positive for the same organism. A contrast-enhanced computed tomography of the chest, abdomen, and pelvis identified a left posterior basal segment consolidation with mild pleural effusion (Fig. 1) and a 19 × 13 mm necrotic lymph node in the aorticpulmonary window. Further investigations demonstrated a decrease in hemoglobin of 3 g/dl, and the peripheral smear exhibited hypochromic, microcytic red blood cells (RBC) with blister cells, few schistocytes (Fig. 2), and thrombocytopenia. Lactate dehydrogenase was high, and haptoglobin was low, while G6PD was surprisingly low (36.7 Mu/109 RBC) (reference range is 224.1–516.9). On further questioning, the patient denied any personal or family history of hemolytic episodes. Ceftazidime continued with fever being subsided on day 10 of hospitalization. His hemoglobin remained stable and repeat blood cultures were negative. In the next days, further laboratory results indicated hyponatremia and deranged liver function tests with cholestatic pattern. Malaria parasite smear was negative, and serology tests for Epstein Bar virus, Dengue virus, herpes simplex virus, hepatitis A, B, C, and E, and HIV were negative. Brucella and leptospira antibodies were negative. Sputum culture, stool for ova and parasites, and urine microscopy were unrevealing. His HBA1C was 13.5% (Table 1). Ultrasound of the abdomen showed mild fatty liver, while magnetic resonance cholangiopancreatography revealed a normal biliary tree, intrahepatic biliary radicals, and common bile duct. Atransthoracic echocardiogram was normal. On day 17 of admission, the patient improved on treatment and was switched to trimethoprim

sulfamethoxazole 960 mg, every 12 h as eradication therapy to prevent relapse of melioidosis (planned for a total duration of 8– 12 weeks). Due to G6PD, the patient was kept in the hospital for 3 more days to ensure that no hemolytic reaction occurred, after which he was discharged. Following discharge, he was followed up through phone twice a week for 2 weeks to inquire about the recurrence of back pain, fatigue, icterus, or any other new symptoms. He was seen 3 weeks after discharge in the infectious disease clinic with no further complaints and reassuring laboratory results, as presented in Table 1.
DISCUSSION
The usual route of infection of melioidosis is close association with soil and exposure through broken skin, inhalation, or ingestion of B. pseudomallei. It is known that certain environmental conditions, including certain occupations (e.g., rice farming), are known to increase the risk of exposure. Melioidosis is rare among Qatari residents. This could be explained by the fact that Qatar is a desert area, devoid of permanent rivers and lakes, with few cultivated areas, and almost all farmers being brought from South Asia or some Arab countries such as Egypt and Sudan. The clinical presentation of melioidosis varies widely, with bacteremia occurring in 55% of patients and 21% of patients developing septic shock [5]. Septicemic melioidosis occurs mostly in patients with underlying diseases, such as renal failure, DM, steroid-treated systemic lupus erythematosus, and antineoplastic therapy [6]. Patients with DM, in particular, have a high incidence of melioidosis, with up to 60% of patients having preexisting or newly diagnosed Type 2 diabetes. Our patient was a farmer with newly diagnosed T2DM. The association with DM may be secondary to impaired neutrophil function with studies demonstrating altered chemotaxis, phagocytosis, oxidative burst, and killing activity [7]. Interestingly, we found that our case had G6PD deficiency, which raised the question, is there a relation between melioidosis acquisition and G6PD? In fact, the relationship between these clinical entities is unclear. However, from a biological point of view, one can assume that there is a causal relationship between them based on the fact that B. pseudomallei stimulates the white blood cells to produce reactive oxygen species (ROS) [1], which can kill the bacteria in a process often referred to as the respiratory burst. ROS are major factors of respiratory burst reactions because their strong activities can destroy microorganisms. They are produced by nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase enzyme through the use of oxygen and NADPH [8]. G6PD converts glucose-6-phosphate to 6-phosphogluconolactone combined with the reduction of NADP to NADPH, a hypothesis proposing that G6PD deficiency reduces NADPH content of WBCs and through this way reduces the production of ROS and the ability to fight against invasive bacteria. However, more studies are needed to elucidate the relation between G6PD deficiency and melioidosis.
The treatment of melioidosis is composed of two phases: The intensive phase and eradication phase. In the intensive phase (10–14 days), ceftazidime or carbapenem is the drug of choice, while in the eradication phase (3–6 months), oral trimethoprim/ sulfamethoxazole is the drug of choice. Therefore, we recommend screening only male patients for G6PD before starting the eradication phase.
CONCLUSION
Melioidosis is a recognized infection in Qatar, which affects people working in agriculture, with DM being the common risk factor. Preventive measures using protective equipment could be particularly useful for risk groups. We recommend screening male patients for G6PD before starting trimethoprim/ sulfamethoxazole, as G6PD deficiency could be a possible risk factor for melioidosis.
AUTHORS’ CONTRIBUTION
Razok A is a member of the treating team who contributed to writing the case history and reviewing the literature. Yousaf Z is a member who contributed to composing and revising the manuscript. LutfAis a member who contributed to composing and revising the manuscript. Alsamawi M is the team leader who contributed to developing the work idea and composing and revising the manuscript.
References
1. Cheng AC, Currie BJ. Melioidosis: Epidemiology, pathophysiology, and management. Clin Microbiol Rev 2007;20:533.
2. Chowdhury FR, Jilani MS, Barai L, et al. Melioidosis in Bangladesh: A clinical and epidemiological analysis of culture-confirmed cases Trop Med Infect Dis 2018;3:40.
3. Melikyan G, Badawi M, Akhtar N, et al. Case of paraspinal collection due to Burkholderia pseudomallei. BMJ Case Rep 2013;2013:bcr2013201447.
4. Al Alousi FS, Al Soub H, El-Shafie SS. Subdural empyema due to Burkholderia pseudomallei. Ann Saudi Med 2000;20:272-3.
5. Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20-year Darwin prospective study. PLoS Negl Trop Dis 2010;4:e900.
6. Limmathurotsakul D, Kanoksil M, Wuthiekanun V, et al. Activities of daily living associated with acquisition of melioidosis in northeast Thailand: A matched case-control study. PLoS Negl Trop Dis 2013;7:e2072.
7. Katz M, Smith S, Conway L, et al. Melioidosis in a patient with Type 1 diabetes mellitus on an insulin pump. Endocrinol Diabetes Metab Case Rep 2018;2018:18-0062.
8. Rostami-Far Z, Ghadiri K, Rostami-Far M, et al. Glucose-6-phosphate dehydrogenase deficiency (G6PD) as a risk factor of male neonatal sepsis. J Med Life 2016;9:34-8.
