Full HTML
Regional Disparities in Covid-19 Vaccine Distribution: A Global Analysis of Manufacturer Contributions and Product Availability
Mostafa Essam Eissa1
Author Affiliation
1 MSc (Pharmaceutical Sciences), Pharmaceutical Scientist | Certified Six Sigma Green Belt, Independent Researcher and Freelance Consultant, Former Inspector in CAPA
Abstract
NO ABSTRACT
DOI: 10.63475/yjm.v4i1.0095
Pages: 193-197
View: 2
Download: 11
DOI URL: https://doi.org/10.63475/yjm.v4i1.0095
Publish Date: 25-05-2025
Full Text
The unprecedented global effort to develop and distribute COVID-19 vaccines has highlighted remarkable scientific achievements and persistent inequities in access across populations. Analyzing of regional vaccine deployment patterns reveals critical insights into pandemic response dynamics, shaped by manufacturing capacity, regulatory frameworks, procurement strategies, and regional health priorities. Data from WHO regions (Africa, the Americas, Eastern Mediterranean, Europe, and Western Pacific) demonstrate significant disparities in vaccine types and suppliers, underscoring the complexity of global vaccination efforts. (Figure 1) illustrates this regional vaccine distribution by WHO regions.
In the Americas WHO Region, the data subset indicates a broad and diverse vaccine ecosystem. The region has seen the presence of numerous vaccine products from a wide array of manufacturers. Notably, established global pharmaceutical giants such as AstraZeneca (with both AZD1222 and Vaxzevria products), Janssen (Ad26.COV 2-S), Moderna (Spikevax), and Pfizer-BioNTech (Comirnaty) feature prominently across a significant number of countries, including Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, and the United States, among many others including Falkland Islands, Anguilla, Antigua and Barbuda. This suggests robust engagement with major international suppliers. However, the data also highlights the presence of vaccines from manufacturers with more regional ties or specific market penetration, such as Beijing CNBG (BBIBP-CorV), Bharat Biotech (Covaxin), CanSino (Convidecia), CIGB (CIGB-66), and Finlay (Soberana Plus, Soberana-02), and even Julphar (Hayat-Vax) and Novavax (NUVAXOVID). The inclusion of vaccines like CIGB-66 and the Soberana series, developed and manufactured in Cuba, and noted in countries like Mexico, Nicaragua, and Venezuela, points to the role of regional scientific capacity and South-South cooperation in vaccine supply. The presence of Bharat Biotech's Covaxin and SII's Covishield also indicates supply streams originating from outside the immediate region, likely through bilateral agreements or international initiatives. The sheer number of different countries listed for the Americas region in this subset suggests a high level of vaccine deployment across a wide geographic area, utilizing a varied portfolio of available vaccines. Moving to the Africa WHO Region, the analysis reveals a diverse but perhaps slightly less expansive range of listed vaccine products and companies compared to the Americas, based on the provided information.
AstraZeneca (AZD1222, Vaxzevria), Beijing CNBG (BBIBP-CorV), Bharat Biotech (Covaxin), CanSino (Convidecia), Gamaleya (Gam-Covid-Vac, Sputnik-Light), Janssen (Ad26.COV 2-S), Julphar (Hayat-Vax), Moderna (Spikevax), Pfizer-BioNTech (Comirnaty), SII (Covishield), and Sinovac (CoronaVac) are noted. The companies involved include AstraZeneca, Beijing Bio-Institute Biological Products (CNBG), Bharat Biotech, CanSino Biologicals, Gamaleya Research Institute, Janssen Pharmaceuticals, Julphar, Moderna, Pfizer-BioNTech, Serum Institute of India, and Sinovac. The distribution spans numerous African nations, including Morocco, Djibouti, Tunisia, Côte d'Ivoire, Algeria, Guinea, Kenya, Rwanda, Seychelles, Zimbabwe, Botswana, Central African Republic, Comoros, Mauritius, Cameroon, Congo, and Togo, among many other nations. Notably, vaccines from Chinese manufacturers (Beijing CNBG, CanSino, Sinovac) and the Serum Institute of India (SII), a major global vaccine producer located in South-East Asia, appear frequently in this African subset. This highlights the crucial role of these suppliers in providing vaccines to the African continent, likely facilitated by mechanisms like the COVAX facility and direct procurement agreements. The presence of Gamaleya's Sputnik vaccines also indicates supply from Russia. While diverse, the mix of vaccines appears somewhat distinct from the Americas, potentially reflecting different procurement priorities, regulatory timelines, or logistical considerations within the region. The single entry for Julphar's Hayat-Vax with another company for Seychelles also points to potentially less common or regionally specific supplies and data reporting variations.
The Eastern Mediterranean WHO Region also exhibits a varied vaccine portfolio. AstraZeneca (Vaxzevria), Beijing CNBG (BBIBP-CorV), Bharat Biotech (Covaxin), CanSino (Convidecia), Finlay (Soberana-02), Gamaleya (Gam-Covid-Vac, Sputnik-Light), Janssen (Ad26.COV 2-S), Moderna (Spikevax), Pfizer-BioNTech (Comirnaty), Shifa (COVIran Barakat), and SII (Covishield) are listed. The associated companies are AstraZeneca, Beijing Bio-Institute Biological Products (CNBG), Bharat Biotech, CanSino Biologicals, Instituto Finlay de Vacunas, Gamaleya Research Institute, Janssen Pharmaceuticals, Moderna, Pfizer-BioNTech, Shifa, and Serum Institute of India. The countries covered include Afghanistan, the United Arab Emirates, Bahrain, Egypt, and Yemen, with many other countries from the region included. Similar to Africa, vaccines from Chinese manufacturers (Beijing CNBG, CanSino, Sinovac), Gamaleya, and SII are prominent. The inclusion of Finlay's Soberana-02 in Iran and Shifa's COVIran Barakat in Iran highlights instances of regional development or specific bilateral partnerships influencing vaccine availability. The presence of a wide range of international suppliers suggests a multi-pronged approach to vaccine acquisition in this region.
In the European WHO Region, there is a particularly large and diverse range of vaccine products and manufacturers across many countries. This region lists vaccines from Anhui ZL (Zifivax), AstraZeneca (AZD1222, Vaxzevria), Beijing CNBG (BBIBP-CorV), Bharat Biotech (Covaxin), Chumakov (Covi-Vac), Gamaleya (Gam-Covid-Vac, Sputnik-Light), Janssen (Ad26.COV 2-S), Moderna (mRNA-1273, Spikevax, and several bivalent Spikevax formulations), Novavax (Covavax, NUVAXOVID), Pfizer-BioNTech (Comirnaty and several bivalent Comirnaty formulations), RIBSP (QazVac), SII (Covishield), Sinovac (CoronaVac), SRCVB (EpiVacCorona), Turkovac, and Valneva (VLA2001), as well as Wuhan CNBG (Inactivated SARS-CoV-2 vaccine). The companies include Anhui Zhifei Longcom Biopharmaceutical, AstraZeneca, Beijing Bio-Institute Biological Products (CNBG), Bharat Biotech, Chumakov, Gamaleya Research Institute, Janssen Pharmaceuticals, Moderna, Novavax, Pfizer-BioNTech, Research Institute for Biological Safety Problems, Serum Institute of India, Sinovac, State Research Center of Virology & Biotechnology, Turkovac, Valneva, and Wuhan Institute of Biological Products (CNBG). The extensive list of countries (Turkmenistan, Uzbekistan, Bosnia and Herzegovina, Albania, Andorra, Armenia, Austria, Azerbaijan, Belgium, Bulgaria, Cyprus, Czechia, Germany, Denmark, Spain, Estonia, Finland, France, with many other nations) reflects the high number of countries within this region and the widespread reporting of vaccine data. The diversity is notable, including vaccines developed within Europe (AstraZeneca, Valneva), the US (Moderna, Janssen, Novavax, Pfizer-BioNTech), Russia (Gamaleya, Chumakov, SRCVB), China (Anhui ZL, Beijing CNBG, Sinovac, Wuhan CNBG), India (Bharat Biotech, SII), and others. The presence of multiple bivalent formulations from Moderna and Pfizer-BioNTech in this region suggests adaptation of vaccine strategies to evolving variants. The inclusion of regionally developed vaccines like QazVac (Kazakhstan), EpiVacCorona (Russia), and Covi-Vac (Belarus), alongside international products, highlights regional manufacturing and supply chains.
Finally, the Western Pacific WHO Region also demonstrates a diverse vaccine landscape. Vaccines from Anhui ZL (Zifivax), AstraZeneca (Vaxzevria), Beijing CNBG (BBIBP-CorV), Bharat Biotech (Covaxin), CanSino (Convidecia), CIGB (CIGB-66), Gamaleya (Gam-Covid-Vac, Sputnik-Light), IMB (Covidful), Janssen (Ad26.COV 2-S), Julphar (Hayat-Vax), Moderna (Spikevax), Novavax (NUVAXOVID), Pfizer-BioNTech (Comirnaty), Shenzhen (LV-SMENP-DC), SII (Covishield), Sinovac (CoronaVac), and Wuhan CNBG (Inactivated SARS-CoV-2 vaccine) are reported. Companies include Anhui Zhifei Longcom Biopharmaceutical, AstraZeneca, Beijing Bio-Institute Biological Products (CNBG), Bharat Biotech, CanSino Biologicals, Center for Genetic Engineering and Biotechnology, Gamaleya Research Institute, Institute of Medical Biology, Janssen Pharmaceuticals, Julphar, Moderna, Novavax, Pfizer-BioNTech, Shenzhen GenoImmune Medical Institute, Serum Institute of India, Sinovac, and Wuhan Institute of Biological Products (CNBG). The region covers countries such as China, Australia, Brunei Darussalam, Federated States of Micronesia, and Tokelau, with many other nations. This region stands out for the significant presence of vaccines developed and manufactured in China (Anhui ZL, Beijing CNBG, CanSino, IMB, Shenzhen, Sinovac, Wuhan CNBG), reflecting China's substantial role as a vaccine producer and supplier within its borders and to neighboring countries. Alongside these, vaccines from AstraZeneca, Bharat Biotech, CIGB, Gamaleya, Janssen, Julphar, Moderna, Novavax, Pfizer-BioNTech, and SII are also present, indicating a mix of regional production and international imports. The inclusion of products like LV-SMENP-DC (Shenzhen) and Covidful (IMB) further highlights domestic or regionally specific vaccine development efforts.
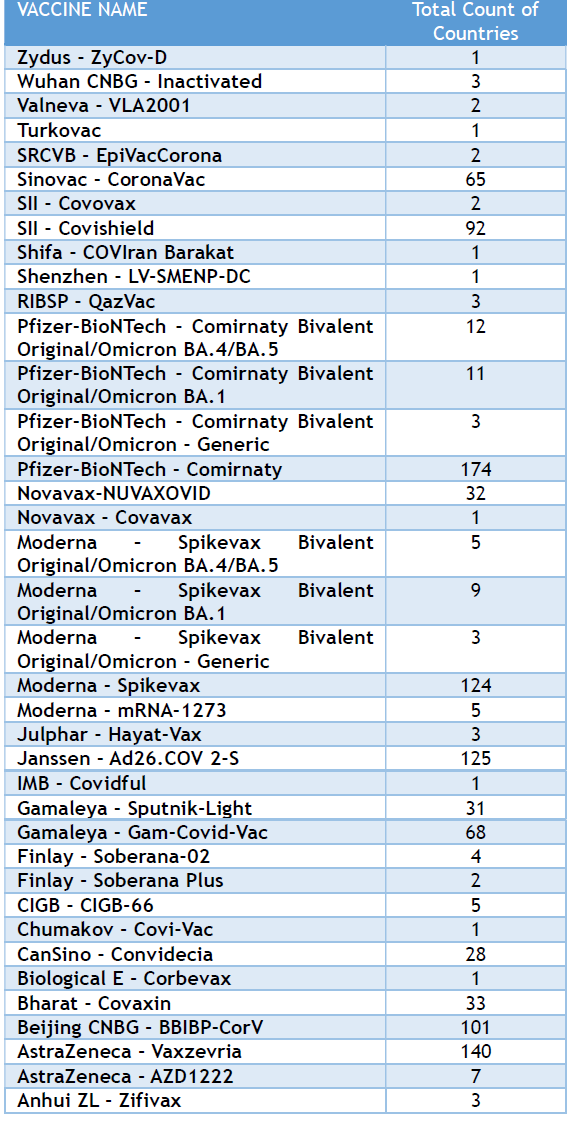
Table 1: Abundance of the common COVID-19 vaccines by countries showing predominance of some companies over others in the global distributions.
Collectively, this regional distribution of vaccines reveals several key patterns and offers a novel perspective on the global vaccine rollout beyond simply total doses administered. Firstly, it highlights the varied portfolio of vaccines available across different WHO regions, influenced by the geographic location of major manufacturers and likely by regional regulatory and procurement dynamics. The Americas and Europe show a strong presence of vaccines from North American and European manufacturers, while Africa, Eastern Mediterranean, and Western Pacific regions demonstrate significant reliance on vaccines from Asia (China, India) and Russia, alongside some Western products. [1] This suggests that geographical proximity to manufacturing hubs and regional economic or political alliances may have played a role in early vaccine access and diversification of supply. [2] Secondly, it underscores the emergence of regionally developed and manufactured vaccines (e.g., CIGB, Finlay, RIBSP, SRCVB, Chumakov, Shifa, IMB, Shenzhen, Wuhan CNBG), which play a role in regional supply, particularly in the Americas, Europe, Eastern Mediterranean, and Western Pacific. [3] This points to the growing capacity and importance of non-traditional vaccine-producing regions in contributing to global health security. [4]
.png)
Figure 1: Distribution of COVID-19 vaccine product counts by WHO region, highlighting the variety and regional presence of different vaccines.
The development and deployment of vaccines like Cuba's Soberana series in the Americas and Iran's COVIran Barakat in the Eastern Mediterranean illustrate how national or regional scientific investment can directly impact local and neighboring vaccine availability. [5] Thirdly, the data implicitly reflects the complexities of data reporting (Julphar - Hayat-Vax in Africa and Western Pacific, Novavax - Covavax in Europe, Turkovac in Europe) and product names (Moderna and Pfizer-BioNTech bivalent generics in Europe), [6] suggesting inconsistencies in how vaccine information is captured and reported globally. [7] This variability in data granularity can pose challenges for comprehensive global analyses of vaccine distribution and impact. [8]
From a scientific standpoint, the observed regional variations in vaccine types could have implications for comparative effectiveness studies, understanding regional differences in immune responses to different vaccine platforms, and evaluating the impact of diverse vaccine strategies on pandemic control within different epidemiological contexts. [9] For instance, regions with a higher prevalence of specific variants might see different real-world effectiveness data depending on the dominant vaccine types used. [10] The reliance on a varied set of suppliers also speaks to the importance of diversified supply chains for pandemic preparedness, reducing dependence on single sources and mitigating risks associated with production delays or export restrictions. [11] The presence of regionally developed vaccines suggests a potential shift towards more distributed vaccine manufacturing capabilities globally, which could enhance future pandemic response equity, provided these vaccines meet international standards for safety and efficacy. [12] This decentralization of manufacturing could be a critical factor in ensuring faster and more equitable access to vaccines in future health crises. [13] Furthermore, the data highlights the different strategies employed for vaccine deployment, from initial product rollouts to the introduction of bivalent formulations targeting specific variants, as seen in the Europe region. [14] Understanding the timelines and regional patterns of these strategic shifts is vital for assessing the evolution of vaccination campaigns. [15] Some companies showed a predominant global presence over others. (Table 1) shows the predominant COVID-19 vaccines, in terms of the number of countries where they are reported, are Pfizer-BioNTech - Comirnaty (174 countries), AstraZeneca - Vaxzevria (140 countries), Janssen - Ad26.COV 2-S (125 countries), Moderna - Spikevax (124 countries), Beijing CNBG - BBIBP-CorV (101 countries), and SII - Covishield (92 countries). These vaccines show the widest reported distribution across the countries.
In conclusion, the brief investigation of vaccination metadata offers a glimpse into the complex and regionally varied ecosystem of COVID-19 vaccine distribution. It highlights the contributions of a global network of manufacturers and underscores the importance of considering regional dynamics, including local production and procurement, when analyzing the global pandemic response. A more comprehensive and standardized global dataset would be invaluable for a deeper scientific understanding of vaccine equity, the effectiveness of different vaccine portfolios in diverse settings, and informing strategies for future global health crises.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
References
- University of Glasgow. Study reveals major gaps in global COVID-19 vaccine access. Univ Glasgow. 2025 Mar 18 [cited 2025 Apr 28]. Available from: https://www.gla.ac.uk/explore/glasgowsocialscienceshub/resources/all/headline_1163770_en.html
- Abu El Kheir-Mataria W, Khadr Z, El Fawal H, Chun S. COVID-19 vaccine intercountry distribution inequality and its underlying factors: a combined concentration index analysis and multiple linear regression analysis. Frontiers in Public Health. 2024 Mar 21;12:1348088.
- RVMC. The time for regionalised vaccine manufacturing has arrived. RVMC. 2025 Jan 13 [cited 2025 Apr 29]. Available from: https://rvmc.net/time-regionalised-vaccine-manufacturing-has-arrived-0
- Africa CDC. Africa's progress towards sustainable local manufacturing health products. Africa CDC. 2025 Feb 11 [cited 2025 Apr 30]. Available from: https://africacdc.org/news-item/africas-progress-towards-sustainable-local-manufacturing-health-products/
- Herder M, Benavides X. 'Our project, your problem?' A case study of the WHO's mRNA technology transfer programme in South Africa. PLOS Glob Public Health. 2024 Sep 23;4(9):e0003173.
- An D, Lim M, Lee S. Challenges for data quality in the clinical data life cycle: systematic review. J Med Internet Res. 2025 Apr 23;27:e60709.
- Kim J, Lee TJ, Hwang SS. Verifying the quality and consistency of immunization monitoring systems. Bull World Health Organ. 2008 Dec;86(12):953-60.
- Inward RP, Jackson F, Dasgupta A, Lee G, Battle AL, Parag KV, et al. Impact of spatiotemporal heterogeneity in COVID-19 disease surveillance on epidemiological parameters and case growth rates. Epidemics. 2022 Dec 1;41:100627.
- Wang X, Pahwa A, Bausch-Jurken MT, Chitkara A, Sharma P, Malmenäs M, et al. Comparative Effectiveness of mRNA-1273 and BNT162b2 COVID-19 vaccines among adults with underlying medical conditions: systematic literature review and pairwise meta-analysis using GRADE. Adv Ther. 2025 Mar 10:1-38.
- Kopel H, Nguyen VH, Bogdanov A, Winer I, Boileau C, Ducruet T, et al. Comparative effectiveness of the bivalent (Original/Omicron BA. 4/BA. 5) mRNA COVID-19 vaccines mRNA-1273.222 and BNT162b2 bivalent in adults with underlying medical conditions in the United States. Vaccines. 2024 Sep 27;12(10):1107.
- Hay M, Teichert A, Kilz S, Vosen A. Resilience in the vaccine supply chain: learning from the COVID-19 pandemic. Vaccines. 2025 Jan 29;13(2):142.
- Medicines Patent Pool. Global mRNA technology transfer programme. MPP. [cited 2025 Apr 27]. Available from: https://medicinespatentpool.org/what-we-do/mrna-technology-transfer-programme
- Bottini Filho L, Abdool Karim S, Fish Hodgson T. Vaccine Inequity in the COVID-19 Crisis: lessons to leverage global health law through market-shaping policies. J Law Med Ethics. 2025 Mar 27;48(S1):17-27.
- Khor SS. New trends in SARS-CoV-2 variants and vaccines. Vaccines. 2025 Mar 3;13(3):265.
- Pavia G, Branda F, Ciccozzi A, Romano C, Locci C, Azzena I, et al. Integrating digital health solutions with immunization strategies: improving immunization coverage and monitoring in the post-COVID-19 era. Vaccines. 2024 Jul 28;12(8):847.
