Full HTML
Budd-Chiari Syndrome in Gaucher Disease Type III in an Adult Libyan Male: Letter to the Editor
Nadya Omran1, Amnna Rayani2, Elmukhtar Habas3
Author Affiliation
1 Gastroenterologist, Department of Internal Medicine, Tripoli University Hospital, Tripoli-Libya
2 Professor/Senior Consultant, Tripoli Children Hospital, Open Libya University, Tripoli, Libya
3 Professor/Senior Consultant, HGH, Open Libya University, Qatar University, Doha, Qatar
Abstract
NO ABSTRACT
DOI: 10.63475/yjm.v4i1.0040
Pages: 189-190
View: 2
Download: 15
DOI URL: https://doi.org/10.63475/yjm.v4i1.0040
Publish Date: 25-05-2025
Full Text
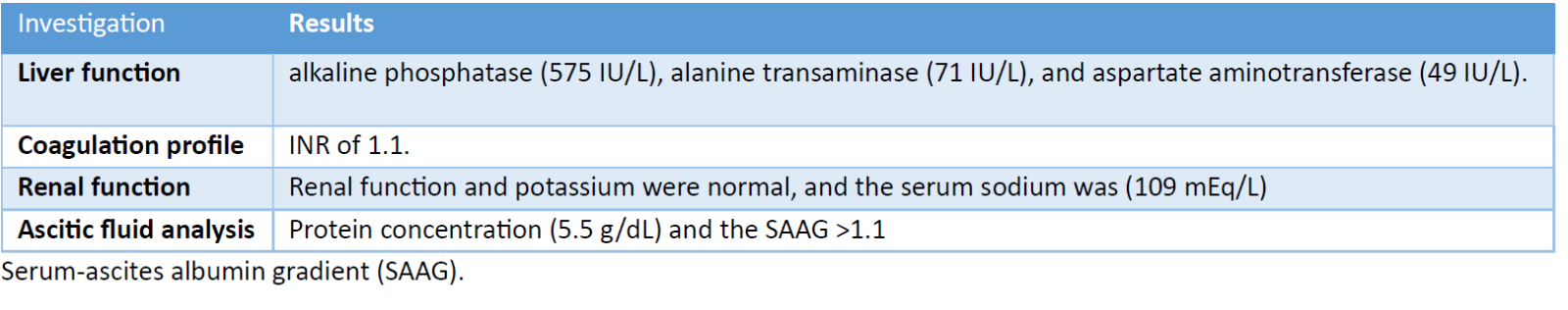
Gaucher Disease (GD) is the most common lysosomal storage disorder. The prevalence of GD is approximately 1/100,000, and type III GD accounts for 5% of cases. [1] It is an autosomal recessive disease due to a GBA gene mutation, leading to glucocerebrosidase enzyme deficiency. [1,2] Gaucher disease (GD) is categorized into three types according to clinical presentation: [3] Type I, which is non-neuronopathic and most common, particularly among Ashkenazi Jews; Type II, which is acute neuronopathic and marked by significant neurological involvement and high mortality rates; and Type III, which is subacute neuronopathic, exhibiting both systemic and neurological symptoms. In this report, we discuss a 24-year-old man from Libya diagnosed with GD type III. His diagnosis was established at the age of one due to symptoms including pallor, poor appetite, and hepatosplenomegaly. Laboratory tests indicated a hemoglobin level of 5.6 g/dL, chitotriosidase activity of 18,742 μmol/L, and an angiotensin-converting enzyme level of 251 UI/L. Genetic analysis confirmed a homozygous L444P mutation. He underwent splenectomy at the age of three, and enzyme replacement therapy (ERT) was administered intermittently with regular follow-ups until 2011. In December 2023, the patient experienced two weeks of abdominal pain, distension, and fatigue. A physical examination revealed ascites, dilated abdominal veins, and an enlarged liver and spleen. Laboratory findings are detailed in Table 1.
Budd-Chiari syndrome was suspected when abdominal ultrasound showed severe liver parenchymal disease, enlarged caudate lobe, and portal vein thrombosis. The initial treatment regimen consisted of furosemide, spironolactone, lactulose, and rivaroxaban. However, anticoagulation therapy was discontinued due to prolonged international normalized ratio (INR). The patient required multiple paracentesis procedures for ascites, which led to an improvement in the symptoms. However, the patient developed recurrent hyponatremia and ultimately passed away three months later.
Neurological symptoms in GD type III typically manifest during childhood or adolescence and include progressive myoclonic epilepsy, cerebellar ataxia, and spasticity. Liver and huge spleen enlargement are characteristic abdominal findings. Pancytopenia, which can manifest as anemia, thrombocytopenia, and skeletal abnormalities, is another presentation.
Hepatic vein outflow obstruction is a rare complication in GD, presenting with ascites, hepatomegaly, and abdominal pain. [4] GD's hepatic vein outflow obstruction occurs due to thrombosis or external compression. [5] GD treatment primarily involves enzyme replacement therapy (ERT), which alleviates systemic symptoms; however, it has limited efficacy on the disease's neurological progression. [6] Management includes anticoagulation, thrombolysis, or surgical interventions such as transjugular intrahepatic portosystemic shunting. Budd-Chiari syndrome is very rare in GD patients and significantly impacts the patient's prognosis. Massive splenomegaly produces pain, compression, and thrombosis. Splenectomy worsens hypercoagulability and recurrent infection, thus, it is advisable to avoid this procedure. Our patient underwent ERT that reduced systemic GD disease symptoms but did not prevent or improve his neurological and thrombotic complications. Treatment of Budd-Chiari syndrome requires anticoagulation and symptomatic treatment.
Table 1. Summary of laboratory results at the presentation of Budd-Chiari syndrome.
In conclusion, the overlap between Gaucher disease type III and Budd-Chiari syndrome is rare, which makes it difficult to diagnose and treat. Effective treatment and early detection of problems improve outcomes. The increased risk of coagulopathy in Gaucher disease, along with the presence of hepatic venous outflow obstruction, hampers the prognosis.
PATIENT CONSENT
A written informed consent was obtained from the patient for publication of this case report.
AUTHORS’ CONTRIBUTION
All authors contributed to the completion of this work. The final manuscript was read and approved by all authors.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
References
- Castillon G, Chang SC, Moride Y. Global Incidence and Prevalence of Gaucher Disease: A Targeted Literature Review. J Clin Med. 2022;12(1):85.
- Omran N, Rayani A, Habas E, Gaucher disease and pulmonary hypertension in adult Libyan female: A case-based literature review. Yemen J Med 2024;3(3):230-4.
- Revel-Vilk S, Shalev V, Gill A, Paltiel O, Manor O, Tenenbaum A, et al. Assessing the diagnostic utility of the Gaucher Earlier Diagnosis Consensus (GED-C) scoring system using real-world data. Orphanet J Rare Dis. 2024;19(1):71.
- Stirnemann J, Belmatoug N, Camou F, Serratrice C, Froissart R, Caillaud C, et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int J Mol Sci. 2017;18(2):441.
- Madir A, Grgurevic I, Tsochatzis EA, Pinzani M. Portal hypertension in patients with nonalcoholic fatty liver disease: Current knowledge and challenges. World J Gastroenterol. 2024;30(4):290-307.
- Gary SE, Ryan E, Steward AM, Sidransky E. Recent advances in the diagnosis and management of Gaucher disease. Expert Rev Endocrinol Metab. 2018;13(2):107-118.
