Full HTML
Spinal Brucellosis Without Apparent Risk Factors: A Case-Based Diagnostic Approach and Literature Review
Elmukhtar Habas1, Abdelaziz Tawengi2, Mohamad Dabbagh2, Ahmad Hamdan3, Mohamed Tawengi2, Muad Fahmi Yousef4
Author Affiliation
1 Consultant, HGH, HMC, Qatar University, Open Libyan University, Doha, Qatar
2 Medicine Department, HGH, HMC, Doha-Qatar
3 Medical Student, Qatar University, Doha-Qatar
4 Medical Student, Department of Medical Radiology, University of Doha for Science and Technology, Doha, Qatar
Abstract
Brucellosis is a zoonotic infection often linked to direct or indirect exposure to animals or unpasteurized dairy products. Atypical presentations in patients without classical risk factors pose significant diagnostic and management challenges. A 68-year-old woman presented with a 2-month history of burning pain involving cervical and lumbar regions, associated with weight loss, decreased appetite, and subjective fever. Examination revealed paravertebral tenderness and left knee swelling with effusion. Previous MRI showed multifocal marrow edema and soft tissue thickening in the lumbar spine, suggesting infection or inflammation. The patient denies exposure to unpasteurized dairy products, raw meat, or animals. Brucella serology revealed positive IgG with Brucella melitensis titer 1:160, later confirmed by blood cultures. PET imaging demonstrated multifocal metabolically active arthritic changes involving the spine, shoulders, and knees. Arthrocentesis confirmed inflammatory arthritis due to brucellosis. The patient was started on doxycycline, rifampin, and a 14-day course of intravenous gentamicin. During hospitalization, management was complicated by persistent knee pain and swelling requiring multimodal analgesia. Spinal brucellosis in the absence of classical risk factors is an odd presentation, emphasizing the importance of maintaining a high index of suspicion in endemic regions. Advanced imaging and microbiological confirmation are pivotal procedures in diagnosis. Early recognition of atypical brucellosis is critical to prevent complications and improve outcomes.
DOI: 10.63475/yjm.v4i1.0103
Keywords: Spinal brucellosis, Arthritis, Magnetic Resonance Imaging (MRI), Antibiotic therapy
Pages: 185-188
View: 2
Download: 5
DOI URL: https://doi.org/10.63475/yjm.v4i1.0103
Publish Date: 25-05-2025
Full Text
Brucellosis is a globally distributed zoonotic disease caused by Gram-negative intracellular bacteria of the genus Brucella. [1] With an estimated annual global incidence exceeding 2 million cases, brucellosis remains a significant public health burden, particularly in endemic regions across the Middle East, Mediterranean basin, Africa, Asia, and Latin America. [2] Brucella melitensis is the most virulent and invasive species affecting humans. It is commonly transmitted through ingesting unpasteurized dairy products or direct contact with infected animals. [3] Clinical presentations are highly variable and often nonspecific, ranging from undulating fever and constitutional symptoms to organ-specific involvement, including osteoarticular, neurologic, and cardiovascular manifestations. [3]
Spinal brucellosis, or brucella spondylitis, occurs in adults and is considered one of the most serious focal forms of the disease. [4] It typically involves the lumbar spine and can lead to significant morbidity if diagnosis and treatment are delayed. Despite the non-existence of established risk factors, such as rural residence, consumption of unpasteurized dairy, or occupational exposure, cases have increasingly been reported among urban populations with no identifiable exposure, increasing diagnostic and therapy challenges. [5] The pathogenesis of spinal brucellosis occurs due to hematogenous dissemination of Brucella species to the intervertebral discs and adjacent vertebrae, resulting in chronic inflammation and tissue destruction. [6] Due to its insidious onset and overlap with other spinal infectious causes and malignancies, its diagnosis often requires a combination of serologic, microbiologic, and imaging modalities, [5] especially when it is not present in the classical presentations.
However, brucellosis without clear risk factors should not underscore the need for heightened clinical suspicion, especially in endemic regions, even without a typical exposure history and clinical presentation. Atypical presentations can result in significant diagnostic delay and mismanagement, emphasizing the importance of integrating epidemiological awareness with comprehensive diagnostic workup. [7] In this report, we present the case of a 68-year-old lady diagnosed with spinal brucellosis without evidence of the typical risk factors for brucellosis.
This is a case of a 68-year-old lady who presented to the hospital complaining of back pain for 2 months. She had been seen abroad for the same complaint and had medical reports that mentioned mild anemia and elevated ESR and CRP. She also presented a magnetic resonance imaging (MRI) report showing multifocal marrow edema and soft tissue thickening at the lumbar spine, suggesting infection or inflammatory processes. The patient had refused to undergo endoscopic biopsy under general anesthesia. The patient was experiencing pain for 2 months, involving the cervical and lumbar regions. The pain had started gradually, and the patient recalls leading a normal life about 3 months earlier. However, the pain had been constant since it started, burning in nature and interrupting her sleep. The pain was non-radiating and associated with decreased appetite, weight loss, and subjective fever. The patient also reported a week of left knee pain and swelling, which was not associated with redness, tenderness, warmth, or skin changes.
Her past medical history was significant for ischemic cardiomyopathy with a history of percutaneous intervention and implantable cardioverter-defibrillator (ICD) placement, left ventricular apical thrombus, and atrial fibrillation on warfarin. She also has hypertension, dyslipidemia, type 2 diabetes mellitus, hypothyroidism, and severe left knee osteoarthritis. She has no recent travel within the past year apart from a 1-month journey to Thailand for a workup of her back pain. She denied any consumption of unpasteurized dairy products, including milk or cheese. She also denied the consumption of undercooked or raw meat or the handling of animals. She lives in a city and denies visiting animal farms or cattle. Her family history was significant for a sister with stomach cancer. She had no known allergies. Physical examination on admission revealed paravertebral tenderness to palpation at cervical and lumbar regions with no step-offs. The left knee was also noted to be swollen, with evidence of small effusion, but it was restricting her mobility.
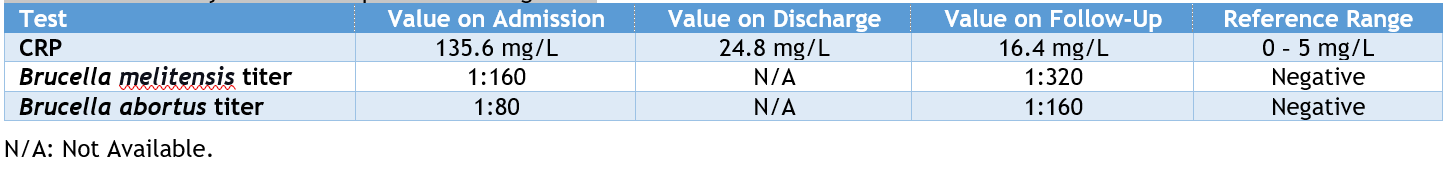
The initial workup showed severe asymptomatic hyponatremia. Her basic lab work was inconclusive toward an etiology. Brucella serology was sent and reported positive for Brucella antibody IgG with negative IgM, with a Brucella abortus titer of 1:80 and a Brucella melitensis titer of 1:160 (Table 1). A day later, blood cultures grew "Gram-negative coccobacilli" suggestive of Brucella species. (Table 1) summarizes the relevant laboratory results.
Table 1: Laboratory results of important investigations
Repeat MRI differed due to the ICD device's incompatibility with the manufacturer's specifications. A PET scan was done, which showed multifocal metabolically active arthritic changes involving the C3-C4 intervertebral disc, superior endplate of T2, and multifocal facet joint arthropathy in the lumbosacral spine (Figure 1 A, B, C, D). The PET scan also reported inflammatory arthropathy of the shoulders and knees (Figure 1).
.png)
Figure 1: A Whole-Body PET-CT scan done using an FDG Tracer. Panel A: Sagittal section of the cervical and thoracic spine; Panel B: Sagittal section of the lumbosacral spine; Panel C: Transverse section showing both shoulders; Panel D: Sagittal section of both knees.
The patient was started on doxycycline, rifampin, and gentamicin after the culture results confirmed the presence of the Brucella melitensis organism. A repeat blood culture after one week of treatment was negative. The patient remained in the hospital to receive a 14-day course of intravenous gentamicin. During her stay, she had a significant increase in her left knee swelling with limited mobility due to pain. Arthrocentesis was conducted, and fluid analysis favored inflammatory arthritis due to brucellosis with the existing spondylitis. During her stay, the management plan was challenging as the patient had severe anorexia and dyspepsia, fluctuating international normalized ratio (INR) levels (she was on anticoagulants for atrial fibrillation), severe left knee pain and swelling despite arthrocentesis, and the present arthralgias requiring several opioid and non-opioid analgesics. On follow-up, the patient reported a resolution of back and joint pains but minimal residual swelling of the left knee. There was a reduction in CRP, yet the titers of both Brucella melitensis and Brucella abortus were on the rise (Table 1).
Human brucellosis, also known as undulant or Malta fever, was first identified in Malta in the 1850s. [8] Brucellosis is the most common zoonotic infection worldwide, [9] with an annual global incidence estimated at approximately 2.1 million cases. [10] Facultative intracellular bacteria of the genus Brucella cause the disease. [11] Among the species, Brucella melitensis is the most pathogenic and invasive, followed by Brucella suis, Brucella abortus, and others. [3] Traditionally, brucellosis is considered a true zoonosis, where humans acquire infection through contact with infected animals or consuming their products. [11] Typical sources include unpasteurized dairy products such as milk and cheese, or handling infected tissues. [12]
Brucellosis is endemic in low- and middle-income countries across the Mediterranean, the Arabian Peninsula, Africa, Asia, and Central and South America. [13] Human-to-human transmission (HHT) is exceedingly rare but has been documented through vertical transmission, breastfeeding, and blood transfusions. [13] The most common Brucella species associated with HHT is Brucella melitensis, identified in 68% of cases, [14] with an incubation period ranging from five days to 6 months. [15] As seen in this case, atypical presentations in urban populations with no clear exposure history challenge the classical epidemiological understanding of brucellosis.
Brucella spp. exploit host immune defenses to establish chronic infections, leading to a spectrum of clinical manifestations, including headaches, recurring fever, migratory joint pain, muscle pain, weakness, loss of appetite, fatigue, general discomfort, sweating, vomiting, diarrhea, abdominal pain, and even miscarriage. More severe complications include endocarditis and neurological disorders. [16, 17] Osteoarticular involvement is the most common complication, including spondylitis, sacroiliitis, and arthritis. Spondylitis is its most prevalent clinical form in adults. [4]
Brucellosis poses numerous diagnostic challenges that significantly hinder public health initiatives. These difficulties are closely related to the extent of individuals' contact with infected animals or their products. [5] Diagnostic tools for brucellosis include blood culture, serological assays, and molecular methods. [18]
Detecting focal infection in brucellosis may be challenging clinically due to nonspecific symptoms and multifocal pain. Different modalities for investigating focal diseases, such as bone scintigraphy, computed tomography (CT), and MRI, are used depending on the suspected site of infection and access to imaging tests. Few case reports reported the contribution of Fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) in diagnosing atypical brucellosis. [19]
Interestingly, in our case, the antibody titers for the two cultured brucella increased at the end-of-therapy. Typically, one would expect a decrease in antibody titers; however, it was observed that no significant correlations existed between the end-of-therapy antibody titers of both Brucella spp. and clinical cure, mortality, length of stay, and duration of therapy. [20]
The patient's unusual presentation highlights the need to consider brucellosis, even when the patient's history and risk factors are unclear. Imaging and serologic tests are essential for confirming or excluding the diagnosis, especially with nonspecific symptoms. Treatment of the case was challenging due to co-existing health conditions, but targeted antibiotics led to gradual improvement. Clinicians should consider brucellosis in patients from endemic areas with back pain, even if systemic symptoms of brucellosis do not coexist.
PATIENT CONSENT
A written informed consent was obtained from the patient for the publication of this case report and all associated images.
AUTHORS’ CONTRIBUTION
All authors have significantly contributed to the work, whether by following the case at the bedside, conducting literature searches, drafting, revising, or critically reviewing the article. They have given their final approval of the version to be published, have agreed with the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None
References
- Herrick JA, Lederman RJ, Sullivan B, Powers JH, Palmore TN. Brucella arteritis: clinical manifestations, treatment, and prognosis. Lancet Infect Dis. 2014;14(6):520-6.
- Al Anazi M, AlFayyad I, AlOtaibi R, Abu-Shaheen A. Epidemiology of Brucellosis in Saudi Arabia. Med 2019;40(10):981-8.
- Hayoun MA, Muco E, Shorman M. Brucellosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 [cited 2024 Feb]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539759/
- Colmenero JD, Reguera JM, Martos F, Sánchez-De-Mora D, Delgado M, Causse M, et al. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore). 1996;75(4):195-211.
- Qureshi KA, Parvez A, Fahmy NA, Abdel Hady BH, Kumar S, Ganguly A, et al. Brucellosis: epidemiology, pathogenesis, diagnosis and treatment-a comprehensive review. Ann Med. 2023;55(2):2295398.
- Rizkalla JM, Alhreish K, Syed IY. Spinal Brucellosis: a case report and review of the literature. J Orthop Case Rep. 2021;11(3):1-5.
- Pu Z, Liu Y, Bai M, Ling T, Pan J, Xu D, et al. Association between diagnostic delays and spinal involvement in human brucellosis: a retrospective case-control study. Open Forum Infect Dis. 2024;11(7):ofae357.
- Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-9.
- Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-86.
- Laine CG, Johnson VE, Scott HM, Arenas-Gamboa AM. Global estimate of human brucellosis incidence. Emerg Infect Dis. 2023;29(9):1789-97.
- Alton GG, Forsyth JRL. Brucella. In: Baron S, editor. Medical Microbiology [Internet]. Galveston (TX): University of Texas Medical Branch at Galveston; 1996 [cited 2024 Feb]. Available from: https://pubmed.ncbi.nlm.nih.gov/21413351/
- Köse Ş, Serin Senger S, Akkoçlu G, Kuzucu L, Ulu Y, Ersan G, et al. Clinical manifestations, complications, and treatment of brucellosis: evaluation of 72 cases. Turk J Med Sci. 2014;44(2):220-3.
- Lai S, Chen Q, Li Z. Human Brucellosis: An Ongoing Global Health Challenge. China CDC Wkly. 2021;3(6):120-3.
- Tuon FF, Gondolfo RB, Cerchiari N. Human-to-human transmission of brucella - a systematic review. Trop Med Int Health. 2017;22(5):539-46.
- Centers for Disease Control and Prevention. Brucellosis reference guide: exposures, testing and prevention [Internet]. Atlanta (GA): 2017 [cited 2024 Feb]. Available from: https://www.cdc.gov/brucellosis/media/pdfs/2025/02/brucellosi-reference-guide.pdf
- Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. Interactions of the human pathogenic brucella species with their hosts. Annu Rev of Microbiol. 2011;65(65):523-41.
- Głowacka P, Żakowska D, Naylor K, Niemcewicz M, Bielawska-Drózd A. Brucella - virulence factors, pathogenesis and treatment. Pol J Microbiol. 2018;67(2):151-61.
- Yagupsky P, Morata P, Colmenero Juan D. Laboratory diagnosis of human brucellosis. Clin Microbiol Rev. 2019;33(1):e00073-19.
- Ghanem-Zoubi N, Kagna O, Dabaja-Younis H, Atarieh M, Nasrallah E, Kassis I, et al. The role of fluorodeoxyglucose positron emission tomography/computed tomography in the management of brucellosis: an observational cohort study. Open Forum Infect Dis. 2023;10(1):ofac704.
- Alsubaie SA, Turkistani SA, Zeaiter AA, Thabit AK. Lack of correlation of Brucella antibody titers with clinical outcomes and culture positivity of brucellosis. Trop Dis Travel Med Vaccines. 2021 Feb 2;7(1):5
