Full HTML
Hemophagocytic Lymphohistiocytosis (HLH) Associated with Mixed Malaria Infection in a Libyan Infant: A rare Case Report
Abdulhakim Alataweel1, Aml Habas2, Elmukhtar Habas3 , Amnna Rayani4
Author Affiliation
1 Specialist, Tripoli Children Hospital, Zawia University, Tripoli, Libya
2 Specialist, Tripoli Children Hospital, Open Libyan University, Tripoli, Libya
3 Professor/Senior Consultant, HGH, Open Libya University, Qatar University, Doha, Qatar
4 Professor/Senior Consultant, Tripoli Children Hospital, Open Libya University, Tripoli, Libya
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a distinct medical condition characterized by symptoms such as fever, hepatosplenomegaly, cytopenia, hypertriglyceridemia, hypofibrinogenemia, and the presence of hemophagocytosis in the bone marrow and other organs. HLH can be classified as either hereditary or secondary, linked to various infections, autoimmune disorders, or cancers. The occurrence of malaria-associated HLH in newborns is considered rare. This report details a case involving a newborn diagnosed with mixed-type malaria complicated by HLH. The diagnosis was delayed because of the rare occurrence of malaria infections in Libya, which the treating clinicians did not initially take into account. The patient received supportive care and antimalarial treatment, which yielded excellent results, and was subsequently discharged from the hospital.
DOI: 10.63475/yjm.v4i1.0109
Keywords: Mixed Malaria, Secondary Hemophagocytic lymphohistiocytosis (HLH), HLH, Malaria-associated HLH.
Pages: 181-184
View: 3
Download: 7
DOI URL: https://doi.org/10.63475/yjm.v4i1.0109
Publish Date: 24-05-2025
Full Text
Hemophagocytic Lymphohistiocytosis (HLH) encompasses a diverse array of conditions marked by systemic hyperinflammation resulting from disrupted immunological homeostasis. It is characterized by infiltrating highly activated lymphocytes and macrophages into tissues, producing significant quantities of proinflammatory cytokines. [1,2] Primary HLH is genetically inherited in an autosomal recessive manner. It often runs in families and typically presents during infancy or early childhood. It is estimated that primary HLH affects about 1 in every 50,000 births. [3] Secondary HLH presents in older children and adults in response to an acute illness trigger rather than an underlying genetic mutation. The incidence of secondary is not known. The most common triggers of secondary HLH include infections (eg, tuberculosis, fungal, and histoplasma both with and without HIV) and malignancy. [3] Additionally, infections from malaria species Vivax and Falciparum are recognized as triggers for secondary HLH-like symptoms. [4] Although Plasmodium malaria in children under five years is well documented, [5] there is little and conflicting evidence on the effect of the disease on newborns under six months of age. Initial research indicated that malaria is infrequent in young children and that clinical manifestations are of little significance. [6] Despite several initial findings indicating a consistent increase in parasite frequency over the first months of life, [7] newborn babies with malaria may have distinct clinical symptoms [8] and reduced parasite density compared with older children. Overall, malaria-associated HLH is uncommon, particularly in newborns. The purpose of presenting this case is to enhance the awareness of healthcare professionals in Libya regarding this rare clinical condition.
The patient was an eight-month-old female from southern Libya. She was born at full term and had been in good health until one month prior to her admission when she developed a high-grade fever that reached 40°C, which did not respond to antipyretics and was accompanied by episodes of shaking. There were no signs of a skin rash or any abnormal movements noted. Two days before her admission, she experienced severe vomiting and diarrhea. The patient had no previous history of similar symptoms and none of her siblings were affected. The family reported that they had not traveled outside of Libya. As a result, she was diagnosed with acute gastroenteritis.
Upon arriving at the hospital, the initial complete blood count (CBC): hemoglobin (Hb) 9.6 gm/dl, MCV 80 fl, white blood cell (WBC) 10,000/ µL, and the platelet count was 29.000/µL. The C-reactive protein (CRP) level was 67 mg/dl. The patient received antibiotics for seven days; however, there was no improvement in the fever, and the overall condition worsened. Consequently, the child was promptly transferred to the pediatric intensive care unit of a tertiary hospital. She displayed symptoms of lethargy, malaise, altered consciousness, fever, severe dehydration, pallor, tachypnea, and discomfort. The child was immediately transferred to a tertiary hospital's pediatric intensive care unit. She exhibited lethargy, malaise, altered consciousness, fever, severe dehydration, pallor, tachypnea, and discomfort. In the intensive care unit, her Hb decreased to 3.8 gm/dl, WBC count was 10.3/ µL, platelet count was 42,000/µL, reticulocyte count was 2.9, with an increase in CRP to 233 mg/dl, serum ferritin at 801 ng/ml, lactate dehydrogenase at 702 IU/l, renal function remained normal, liver function tests indicated GPT at 43 IU/l, GOT at 87 IU/l, total bilirubin at 0.6, and negative viral screening. The first blood film indicated normocytic normochromic anemia, exhibiting signs of hemolysis and lymphocytosis, with no aberrant cells observed upon three repetitions, rendering it uninformative. The first abdominal ultrasound did not reveal any abnormalities or hepatosplenomegaly. In the pediatric critical care unit, the child was administered antibiotics (cefotaxime and vancomycin at anti-meningitis dosages), dexamethasone, and a blood transfusion. She remained in the intensive care unit for three days before being transferred to the pediatric ward, where she underwent many transfusions of blood and platelets owing to declining hemoglobin and platelet levels of unknown origin. The patient remained febrile and had hematochezia. On the eighth day of hospitalization, the belly had grown bloated, and the patient had hepatosplenomegaly. D-DIMER 12 mg/L, fibrinogen 227 mg/dL. The coagulation profile was normal: serum ferritin, 3000 ng/ml; erythrocyte sedimentation rate (ESR), 20; Vitamin B12 was 804.3 pg/ml, and serum albumin, 3.15 g/dl. The fasting lipid profile revealed triglyceride, LDL, and cholesterol levels of 868 mg/dl, 16 mg/dl, and 122 mg/dl, respectively. The most recent blood film indicated a mixed malarial infection (P. falciparum and P. malariae); a malarial antigen test was conducted and yielded a positive result for mixed malaria infection. A conclusive diagnosis of malaria-associated secondary HLH was made. A review of the patient's history indicated that the family and the infant resided near a refugee camp that housed individuals from Africa. There is a possibility that immigrants may have transported an infected female Anopheles mosquito in their clothing or belongings.
Management and outcome
The malaria treatment protocol comprised intravenous Artesunate at a 3 mg/kg dosage for three doses (0, 12h/24h), accompanied by monitoring hepatic and renal functions. Subsequently, Coartem (Artemether + Lumefantrine) was administered in 20 mg tablets for five doses (0, 8h, 24h, 36h, 48h, 60h). Due to the high suspicion of bacterial infection and septicemia, the patient received intravenous dexamethasone, and broad-spectrum antibiotics (meropenem 10 mg/kg every 8 hours, and amikacin 7.5 mg/kg every 12 hours).
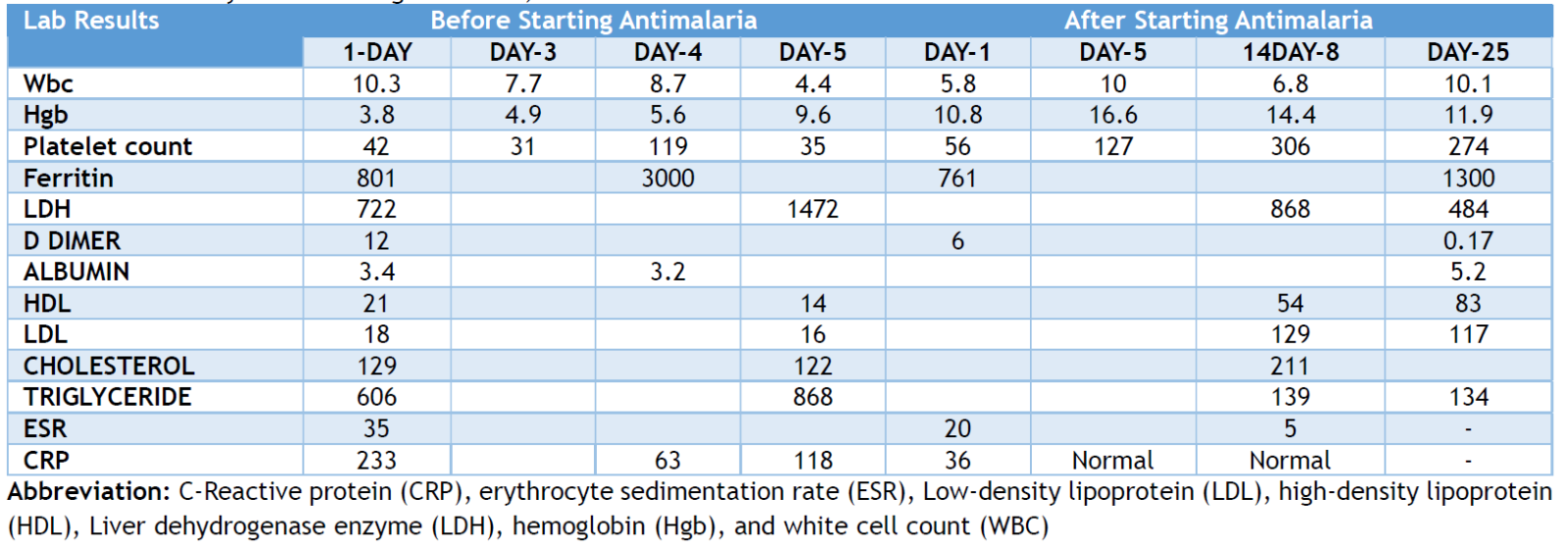
Since hemophagocytic HLH is secondary to malaria, HLH treatment was not required. After five days of treatment, the patient exhibited no fever or further decline in hemoglobin or platelet levels. The patient was discharged and subsequently followed up in the hematology outpatient department. The blood film was repeated after one month, revealing ringed gametocytes. Oral Primaquine for malaria was recommended but was unavailable and hence not administered. The family had a second consultation abroad and was advised that there was no need for gametocyte therapy. All features of HLH were resolved after one month, and her CBC was normal, with a final Hb 11.9 mg/dl, platelet count 274,000/µL, white blood cell count 10.1/µL, D-dimer 0.17 ng/dl, triglyceride 130 mg/dl and liver and renal function tests were normal. The laboratory results obtained before and after antimalarial therapy are detailed in Table 1 and Figure 1. Table 1. summarizes the Laboratory results in the tertiary care hospital, before and after antimalarial treatment.
.png)
Figure 1. Blood film and malaria P. falciparum/Pan test
Table 1: Laboratory results during admission, before and after antimalarial treatment.
Secondary HLH is uncommon in newborns and typically occurs in conjunction with infections, lymphoid malignancies, or connective tissue disorders. The pathophysiology underlying secondary HLH is still not fully understood, and the condition often remains undetected. This case report encompasses several issues that warrant further exploration.
First, although Libya was considered free of local malaria transmission in 2012 (representing 1.0% of reported cases in malaria-free countries), [8] the risk of malaria reintroduction into Libya is increasing. This is due to the increasing number of imported malaria cases caused by immigration to Libya from malaria-endemic countries, as well as the lack of effective health policy and planning due to the civil war. This case may be a warning sign that malaria is endogenous in Libya and further efforts are needed to investigate the current situation of the malaria epidemic in all regions of Libya. Therefore, malaria infection must be considered in newborns who present with fever that is refractory to antibiotics and antipyretics.
Second, the idea that secondary HLH, particularly malaria-associated HLH, is uncommon in newborns should not be relied upon. It appears that secondary HLH can impact individuals of any age group, and the lack of recognition and reporting in resource-limited environments attributed to lack of facilities, may lead to its perceived rarity. Our case represents the first documented instance of malaria-associated HLH in Libya; however, we suspect that numerous cases have gone unrecognized due to a low index of suspicion. Therefore, a heightened level of suspicion is essential for accurate diagnosis, particularly in patients who present with unexplained fever and elevated ferritin levels, necessitating thorough investigations.
Third, there is a lack of level I evidence from which the best management plans for malaria-associated HLH may be derived. In fact, the most evidence is based on case reports and series. In a recent case series and literature review, [9] the authors found that, only 41% of patients received specific treatment for malaria-associated HLH. The mortality rate was 5%, which is lower compared to the rate reported for secondary HLH triggered by other infectious diseases. Additionally, a case report of P. falciparum reported that rapid response was observed after treatment by antimalarial agents, immunomodulatory therapy, and supportive care.[10] In our case, only supportive care and antimalarial therapy were used without immune therapy, resulting in an excellent outcome.
Timely and precise diagnosis of malaria infection, along with effective treatment for malaria, is crucial for patient survival, even in cases accompanied by HLH. Healthcare professionals must recognize that malaria infection is possible in Libya.
PATIENT CONSENT
A written informed consent was obtained from parents for the publication of this case report and all associated images.
AUTHORS’ CONTRIBUTION
All authors have significantly contributed to the work, whether by following the case at the bedside, conducting literature searches, drafting, revising, or critically reviewing the article. They have given their final approval of the version to be published, have agreed with the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
References
- Wu Y, Sun X, Kang K, Yang Y, Li H, Zhao A, et al. Hemophagocytic lymphohistiocytosis: current treatment advances, emerging targeted therapy and underlying mechanisms. J Hematol Oncol. 2024;17(1):106.
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med. 2012;63:233–46.
- Philadelphia TCH of. Hemophagocytic Lymphohistiocytosis (HLH) [Internet]. The Children’s Hospital of Philadelphia; 2014 [Accessed May 2025]. Available from: https://www.chop.edu/conditions-diseases/hemophagocytic-lymphohistiocytosis-hlh/.
- WHO: World malaria report. 2011, World Health Organization, Geneva
- Macdonald G. The analysis of malaria parasite rates in infants. Trop Dis Bull. 1950, 47: 915-38.
- Brabin B. An analysis of malaria parasite rates in infants: 40 years after Macdonald. Trop Dis Bull. 1990;87: 1-21.
- Sehgal VM, Siddjiqui WA, Alpers MP. A seroepidemiological study to evaluate the role of passive maternal immunity to malaria in infants. Trans R Soc Trop Med Hyg. 1989, 83:105-6.
- libya_2012.pdf [Internet]. [Accessed 2025 Feb]. https://www.emro.who.int/images/stories/rbm/documents/malaria_profiles_2012/libya_2012.pdf?ua=1.
- Orth HM, Wiemer D, Schneitler S, Schönfeld A, Holtfreter MC, Gliga S, et al. Hemophagocytic lymphohistiocytosis-how common and how severe is it as a complication of malaria? Retrospective case series and review of the literature. Infection. 2024;52(2):471-482.
- Zhou X, Duan ML. Malaria-associated secondary hemophagocytic lymphohistiocytosis: A case report. World J Clin Cases. 2021 Aug 6;9(22):6403-6409.
