Full HTML
Bleomycin Pulmonary Toxicity in Patients with Germ Cell Tumors Treated with Bleomycin Containing Regimens: Experience with 4 Cases
Mohammed Sadiq Ahmed1, Mohammad Abdel Daem Yassin2, Rawan Ahmed Mohammed3, Aveen Sadiq 4, Abdurahman Mustafa Kday5
Author Affiliation
1 Consultant, Ambulatory Medicine, Hazm Mebaireek General Hospital, Doha, Qatar
2 Senior consultant, Hematology department, National Center for Cancer Care and Research, Doha, Qatar
3 Pharmacist, Oncology department, National Center for Cancer Care and Research, Doha, Qatar
4 Medical student, Royal college of surgeons-Medical College of Bahrain, Bahrain
5 Resident, Department of Medicine, Hamad General Hospital, Qatar
Abstract
Background: Information on Bleomycin pulmonary toxicity (BPT) in Qatar is scarce. The aim of this study was to estimate the prevalence of BPT and to describe its clinical significance and outcome in germ cell tumor (GCT) patients who received bleomycin-containing regimens.
Methods: This retrospective cross-sectional study was conducted at the National Center for Cancer Care and Research. It included all patients diagnosed with GCT and treated with a bleomycin-containing regimen between January 2002 and December 2008
Results: We identified fourteen patients with GCT who received bleomycin containing regimen. Four of them (28.5%) had developed BPT, and they were males with mean age of 39.3±8.3 years (range: 25-46 years). The calculated creatinine clearance before treatment was normal in the 4 cases. Evaluation of the chest computed tomography scan before starting bleomycin containing regimens revealed that none of our patients had pre-existing parenchymal lung disease. The mean cumulative bleomycin dose was 187.5± 153.7 U, while the mean time to onset of BPT was 3.5±2.1 months. Once the diagnosis of BPT was established, bleomycin was discontinued in the four patients and short courses of dexamethasone were administered. Two patients (50%) died, while one patient survived with a fibrosis sequel, and the fourth patient recovered without a fibrosis sequel.
Conclusion: BPT is one of the life-threatening side effects of this drug that every doctor should be aware of when treating GCT, therefore, a high index of suspicious is needed for early recognition of BPTs.
DOI: 10.63475/yjm.v4i1.0004
Keywords: Bleomycin, pulmonary toxicity, Germ cell tumor, corticosteroids
Pages: 170-173
View: 4
Download: 6
DOI URL: https://doi.org/10.63475/yjm.v4i1.0004
Publish Date: 23-05-2025
Full Text
Bleomycin is a glycopeptide antineoplastic antibiotic, that has been widely used since the early 1970s. The drug is poorly absorbed when administered orally and is generally administered intravenously, where <1% is bound to plasma proteins. The kidneys excrete between 50 and 70 percent of the drug unchanged and the drug half-life in patients with normal renal function is 2–5 hours, which can extend to 30 hours with reduced glomerular filtration rates (GFRs). [1] Pulmonary toxicity, which can be life-threatening, is the main disadvantage of treatment with bleomycin and has been reported in up to 29% (range: 8-29%) [2,3] of patients receiving this drug and carries a 10%‐20% risk of mortality. [4]
A wide range of pulmonary bleomycin toxicity has been reported as a complication of such therapy, the most common variant of which is bleomycin-induced pneumonitis, which may eventually progress to fibrosis. [5,6] The toxicity mechanism of bleomycin is unclear and is likely to include oxidative damage, the release of inflammatory cytokines, a bleomycin hydroxylase enzyme deficiency in the lungs, and genetic susceptibility.[5] There are a variety of factors that increase the risk of developing bleomycin-induced pulmonary toxicity, including increased age, high cumulative doses of bleomycin, renal impairment (creatinine clearance <35 ml/min), high concentration oxygen therapy, and chest radiotherapy.[6]
In Qatar, we used this drug in the treatment of GCT; however, few reports highlighted the prevalence of this clinical entity and its clinical importance. In this series, we aimed to report the prevalence of BPT and to describe its clinical significance and outcome in germ cell tumor (GCT) patients who received bleomycin-containing regimens.
This retrospective cross-sectional study was conducted at the National Center for Cancer Care and Research (NCCCR). It included all patients diagnosed with germ cell tumors (GCT) and treated with a bleomycin-containing regimen between January 2002 and December 2008.
In Qatar, NCCR is the premier cancer center. It is part of Hamad Medical Corporation (HMC) and cares for patients with cancer who need ongoing therapies such as chemotherapy and radiotherapy. NCCCR also treats blood diseases and will open the first Bone Marrow Transplantation Center in Qatar soon. Our primary endpoint was to identify cases of BPT and in-hospital mortality.
The diagnosis of BPT was established by the combination of systemic symptoms, and radiological (chest Computed Tomography (CT) scan) findings, while other disorders should be excluded. Histological findings on biopsies or lavages were used to support the diagnosis, whereas pulmonary function tests were not performed. This study has been approved by the medical research committee at HMC. The data obtained from our NCCCR database were analyzed by using simple statistics. The results of analyses of continuous variables are expressed as means and standard deviations (SD) unless otherwise specified.
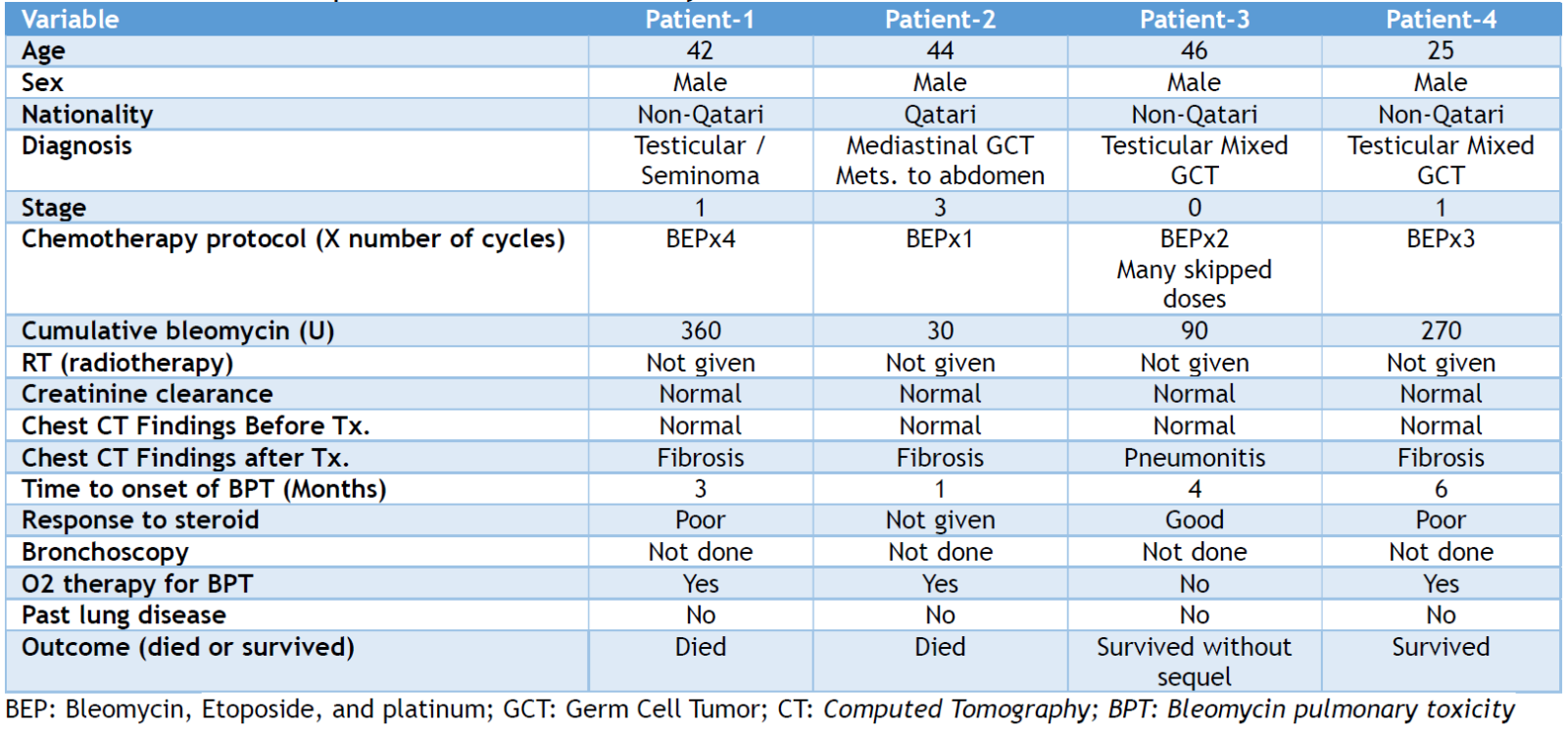
During the study period, we found fourteen patients with GCTs who received a bleomycin-containing regimen. Four of them (28.5%) had developed BPT, three of which developed fibrosis, while one patient had pneumonitis. All patients with BPT were males with a mean age of 39.3±8.3 years (range: 25-46 years).
The calculated creatinine clearance before treatment initiation was normal in the 4 cases and evaluation of the chest CT scan before starting bleomycin-containing regimens revealed that none of our patients had pre-existing parenchymal lung disease or fibrosis. The mean cumulative bleomycin dose was 187.5± 153.7 U (range: 30-360 U), while the mean time to onset of BPT was 3.5±2.1 months (range: 1-6 months). None of our patients received concurrent oxygen supplements or chest radiotherapy. The pre-treatment pulmonary function tests showed that none of the four patients had moderate to severe restrictive lung disease. Once the diagnosis of BPT was established, bleomycin was discontinued in the four patients and short courses of dexamethasone were administered. Of all, two patients (50%) died, while one patient survived with a fibrosis sequel, and the fourth patient recovered without a fibrosis sequel. Table 1 describes the demographic and clinical aspects of the patients involved in this study.
Table 1. Characteristics of patients involved in this study
This study is an attempt to draw the attention of our physicians to BPT as a therapeutic complication that may increase mortality or would make the patients live with these sequelae for the rest of their lives. Early recognition of the BPT and identification of their risk factors may reduce the number of patients who develop this unwanted complication, as the condition is almost entirely irreversible once the fibrosis progresses to an acute respiratory compromise. [4,5]
Based on the criteria used for the diagnosis, the incidence of BPT varies from population to population and from study to study in the same country. As noted in our series, the prevalence of BPT among our patients was 28.5%, which falls within the international range of 8-29% that is mentioned in the literature. As 70% of the drug is excreted through the kidney, close monitoring of the renal function is mandatory. Renal impairment due to low GFR may increase the drug's half-life leading to longer exposure of the lungs and consequently, increase the risk of BPT.[1,7] In our series, the calculated creatinine clearance was normal in all patients before initiation of the bleomycin-containing regimen.
It has long been assumed that high cumulative doses of bleomycin > 400 IU increase the risk of lung toxicity, however, few reports have found that lung toxicity is not dose-related and can occur even with a cumulative dose of <50 units. [8-11] In our patients, the cumulative dose of bleomycin was between 30 and 360 IU, indicating that BPT could occur regardless of the cumulative dose of bleomycin.
Age over 40 has been found to be associated with a higher risk of BPT compared to patients under 40 in the treatment of germ cell tumors. [1] Our cases are no exception; three of our patients were over 40 years of age. The available pieces of evidence suggest that concurrent use of G-CSF, high-concentration oxygen supplementation, thoracic irradiation, and concurrent high-dose cisplatin administration increase the risk of bleomycin lung toxicity. [1,4,10] In contrast, none of our patients had simultaneously used these agents during or before treatment. Many reports have shown that BPT usually evolves progressively during treatment and clinical manifestations occur between one and six months after bleomycin administration, although the development of BPT has also been recorded for up to two years after discontinuation of bleomycin. [10,11] Similarly, BPT occurred among our patients within 1-6 months.
There is a controversy regarding the utilization of pulmonary function tests (PFT) in diagnosing or predicting BPT. While Zhao et al. [7] found PFT useful in diagnosing BPT, other researchers such as Roncolato et al. [12] and McKeage et al. [13] reported that the use of PFTs in predicting BPT should be questioned. At our center, we do not use PFT to diagnose BPT as it is a controversial issue. Therefore, none of our patients had undergone PFT and the diagnosis was based on clinical and chest imaging (chest CT findings).
There are few reports to guide the management of BPT. [14] If BPT is being considered, discontinuation of treatment may reverse lung damage, while continued treatment with bleomycin may lead to worsening the toxicity. [14] Some agents have been tried to treat BPT. Although no randomized trials have been performed, observational studies have indicated a beneficial effect of corticosteroids. [15] Therefore, until further data are known, permanent discontinuation of bleomycin is currently the mainstay of treatment, in addition, glucocorticoids can be used if they are not contraindicated. In the present study, bleomycin was discontinued in the four patients once BPT was considered, and short courses of dexamethasone were administered, while oxygen was supplemented as needed for the patients with hypoxemia to keep oxygen saturation at an optimal level.
Mortality from BPT in our study was 50%, which is above the 10-20% range [10] reported in the literature. The reason for this finding is unclear. As mentioned earlier, one of our patients who was on corticosteroids recovered without a sequel. Whether corticosteroid therapy played a role in his recovery is not clear.
The main limitations of this study include the small sample size, which is insufficient to demonstrate the statistical significance of the risk factors associated with BPT, the retrospective design of this study, and the outdated timeframe of the study.
BPT is one of the life-threatening side effects of this drug that every doctor should be aware of when treating GCT. A high index of suspicion is needed for early recognition of BPTs, which should be considered in any patient with new or progressive respiratory complaints associated with certain factors like age >40. Moreover, we found that the prevalence of BPT is comparable to international findings.
AUTHORS’ CONTRIBUTION
Each author has made a substantial contribution to the present work in one or more areas including conception, study design, conduct, data collection, analysis, and interpretation. All authors have given final approval of the version to be published, agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
References
- O'Sullivan JM, Huddart RA, Norman AR, Nicholls J, Dearnaley DP, Horwich A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol. 2003;14:91-96.
- Kwan EM, Beck S, Amir E, Jewett MA, Sturgeon JF, Anson-Cartwright L, et al. Impact of granulocyte-colony stimulating factor on bleomycin-induced pneumonitis in chemotherapy-treated germ cell tumors. Clin Genitourin Cancer 2017. pii: S1558-7673 (17) 30267-7.
- Maruyama Y, Sadahira T, Mitsui Y, Araki M, Wada K, Tanimoto R, et al. Prognostic impact of bleomycin pulmonary toxicity on the outcomes of patients with germ cell tumors. Med Oncol 2018;35(6):80
- Ge V, Banakh I, Tiruvoipati R, Haji K. Bleomycin-induced pulmonary toxicity and treatment with infliximab: A case report. Clin Case Rep. 2018;6: 2011-2014.
- Sleijfer S. Bleomycin‐induced pneumonitis. Chest. 2001;120:617‐624.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb 2010; 40:213–5
- Zhao Q, Cao D, Yu M, Yang J, Liu Y, Xiang Y, et al. Safety and efficacy of bleomycin/pingyangmycin-containing chemotherapy regimens for malignant germ cell tumor patients in the female genital system. Oncotarget. 2017;8(9):15952-15960.
- Liu T, De Los Santos FG, Phan SH. The Bleomycin model of pulmonary fibrosis. Methods Mol Biol. 2017;1627:27‐42.
- Iacovino JR, Leitner J, Abbas AK, Lokich JJ, Snider GL. Fatal pulmonary reaction from low doses of bleomycin. An idiosyncratic tissue response. JAMA 1976;235:1253-5
- Reinert T, Baldotto CSdR, Nunes FAP, Scheliga AAdS. Bleomycin-induced lung injury. J Cancer Res 2013; 2013:9
- Barnardt Pand, Griffith-Richards S. Ovarian germ cell tumour and bleomycin-induced lung injury. Southern African Journal of Gynaecological Oncology 2018; 10:30–33.
- Roncolato FT, Chatfield M, Houghton B, Toner G, Stockler M, Thomson D, et al. The effect of pulmonary function testing on bleomycin dosing in germ cell tumours. Intern Med J 2016;46:893-8.
- McKeage MJ, Evans BD, Atkinson C, Perez D, Forgeson GV, Dady PJ. Carbon monoxide diffusing capacity is a poor predictor of clinically significant bleomycin lung. New Zealand Clinical Oncology Group. J Clin Oncol 1990;8:779-83.
- Watson RA, De La Peña H, Tsakok MT, Joseph J, Stoneham S, Shamash J, et al. Development of a best-practice clinical guideline for the use of bleomycin in the treatment of germ cell tumours in the UK. Br J Cancer. 2018;119:1044-1051.
- White DA, Stover DE. Severe bleomycin-induced pneumonitis. Clinical features and response to corticosteroids. Chest 1984;86:723-8.
