Full HTML
Tertiary Hospital Experience in Outpatient Treatment of Infantile Hemangiomas: A Prospective Study
Amnna Rayani1, Najwa Alkrikshi2 , Abdulhakim Alataweel2, Fatma Abuzaid2, Aml Habas3, Elmukhtar Habas4
Author Affiliation
1 Professor/Senior consultant, Tripoli Children Hospital, Open Libyan University, Tripoli-Libya
2 Consultant, Department of Pediatrics, Tripoli Children Hospital
3 Specialist, Tripoli Children Hospital, Open University, Tripoli-Libya
4 Professor/Senior consultant, Open Libyan University, Tripoli-Libya
Abstract
Background: Infantile hemangioma (IH) is observed at varying frequencies among children, impacting 10% of infants. The majority of uncomplicated IH cases experience spontaneous involution, while a minority necessitate intervention. This study aimed to assess the safety and effectiveness of propranolol in treating IH in Libyan pediatric patients.
Methods: From 2013 to 2016, a total of 100 patients diagnosed with infantile hemangioma (IH) were monitored at a pediatric tertiary hospital's hematology clinic. Their demographic and clinical information, along with digital images of the lesions, were collected prospectively to evaluate coloration, size, and improvement of the hemangioma lesions. Following parental consent to initiate treatment, a regimen of oral propranolol, atenolol, or a combination of propranolol and steroids was commenced.
Results: The study included 100 children diagnosed with infantile hemangioma (IH), consisting of 62% females and 38% males. Out of these, 68 children were treated exclusively with propranolol, while 12 were started on atenolol. The remaining 20 children received a combination of steroids and propranolol. Hemangioma lesions were primarily located on the face (56%). After an average follow-up duration of 10.53 ±7.21 months, among the 68 children treated solely with propranolol, 12 under the age of 12 achieved complete resolution of their hemangiomas, 15 demonstrated near-complete resolution, and the remaining 41 showed a significant reduction in hemangioma size without considerable disfigurement. Following a six-month discontinuation of propranolol, 2 children from this group experienced a relapse of their lesions. In one instance, the lesion resolved after 12 months of resuming propranolol, while the other required 18 months of treatment. Among the children treated with atenolol, two attained complete resolution, two had near-complete resolution, and 8 exhibited a significant reduction in hemangioma size. No relapses or changes in lesion size were noted after the cessation of atenolol.
Conclusion: Oral propranolol is considered both safe and effective for the treatment of IH at a daily dosage of 2 mg/kg, with no significant adverse effects reported. Atenolol serves as an alternative to propranolol, while steroids, despite their potential side effects, have been shown to improve lesions in certain pediatric patients. Nevertheless, further large multicenter studies are necessary.
DOI: 10.63475/yjm.v4i1.0033
Keywords: Propranolol, Infantile Hemangioma, Angiogenesis, Infantile Hemangioma Therapy
Pages: 146-152
View: 4
Download: 10
DOI URL: https://doi.org/10.63475/yjm.v4i1.0033
Publish Date: 23-05-2025
Full Text
The most prevalent benign vascular tumor in infants is an infantile hemangioma (IH), which occurs in approximately 4% to 5% of newborn babies. [1] These types of vascular lesions are also known as "Strawberry" lesions. Hemangiomas come in various forms. Those present at birth are known as congenital hemangiomas, while infantile hemangiomas develop in the weeks or months following birth. The latter type is distinguished by an initial phase of rapid expansion, followed by a period of natural regression.[2] The underlying causes of IHs remain largely unknown, though several theories exist. The prevailing hypothesis proposes that oxygen deprivation increases the expression of glucose transporter-1 (GLUT1) and vascular endothelial growth factor, triggering the movement of endothelial progenitor cells characterized by increased CD133 and CD31 markers. [3] An alternative theory suggests that increased hemangioma stem cells originate from placental trophoblasts [4], promoting vascular growth. Another perspective suggests that the development of infantile hemangioma involves both vasculogenesis (the creation of new blood vessels from progenitor cells) and angiogenesis (the formation of new vessels from existing ones). [3] It has been proposed that angiogenic factors stimulate endothelial cells and pericytes to initiate the development of a capillary network. [5] These theories are summarized in (Figure 1).
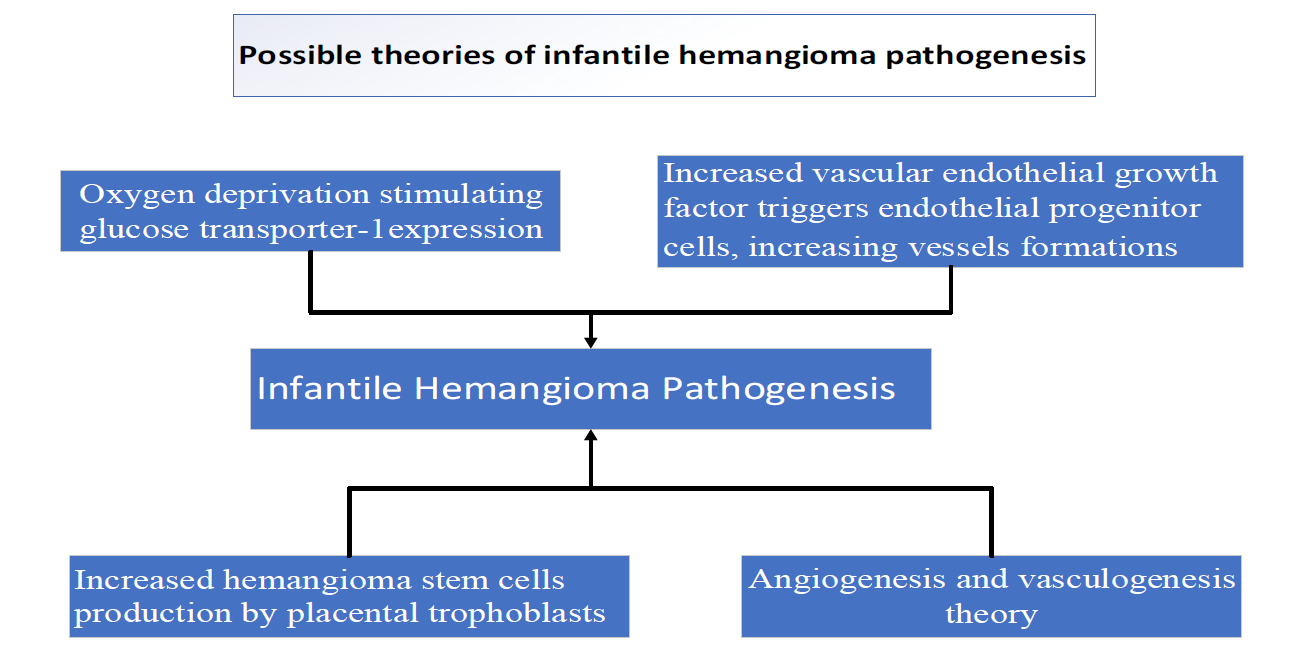
Figure 1: Summarizes the possible mechanisms of infantile hemangioma pathogenesis
Hemangioma is primarily found in the head and neck, although it can also affect other sites. Hemangioma lesions are usually painless. However, infantile hemangioma may ulcerate, bleed, and develop a secondary infection. [6,7] Steroids were the first line of treatment before the propranolol era. [8] Nevertheless, steroid adverse effects, such as weight gain, cushingoid facies, hypertension, adrenal suppression, hyperglycemia, immunosuppression, gastric irritation, behavioral changes, bone complications, and transiently reduced longitudinal growth, make their use as a first-line type of treatment an unfavorable option. Recently, it has been reported that propranolol has higher efficacy than steroids for treating IHs, providing the basis for propranolol use as an alternative to steroids. [9]
Propranolol is a non-selective Beta-blocker that has been used for a long time in treating hypertension, portal hypertension, and the prevention of arrhythmia. Recently, it was reported that propranolol can rapidly and significantly reduce hemangioma lesions without significant lethal adverse effects. [10] Propranolol treats large segmental hemangiomas of the trunk and extremities and large hemangiomas of the face. [11,12] Propranolol successfully treated IH for 4-8 months, [13] and even until the end of the proliferation phase, or even up to complete resolution.
The precise mechanism by which propranolol affects the hemangioma lesion is unknown; different mechanisms have been suggested. Storch and Hoeger suggested that propranolol interferes with the function of endothelial cells, including vascular tone, angiogenesis, and apoptosis [11] by downregulation of vascular endothelial growth factor expression in hemangioma. [12,14] Propranolol administration can reduce the effects of vasoconstriction, angiogenesis inhibition, apoptosis induction, and the central pathway of β-blockade, which predisposes to IH. [12] Propranolol's multifaceted action makes it superior to previous therapies (e.g., corticosteroids).
Propranolol is contraindicated in premature infants within two weeks of birth, infants with obstructive bronchitis, anomalies of the central nervous system, and infants with abnormal renal function. [15] Bradycardia, hypotension, bronchospasm, wheezing, hypoglycemia, and electrolyte abnormalities are all potentially significant side effects of propranolol. [12,13] These side effects are infrequently recorded and are usually minor and temporary. [12,13] Other rarely reported propranolol adverse effects include sleep disturbances (e.g., restless sleep, nightmares) and gastrointestinal discomfort. Propranolol therapy is usually beneficial when initiated early in the proliferation phase. A study noted that Oral beta-blocker therapy has proven highly effective and well-tolerated in treating infantile hemangiomas.
The treatment yields positive results in over 94% of patients, with 90% experiencing resolution of the primary functional issue that prompted the intervention. [16] A study of 311 hemangioma patients concluded that treatment of IH with oral beta-blocker therapy is highly effective and well tolerated. More than 94% of patients responded to treatment, and 90% showed resolution of the primary functional indication for treatment. The same study reported that switching to another beta-blocker agent produces a good response without problem. [17]
Recent articles indicated that propranolol is considered a first-line treatment with fewer side effects. However, there is still debate surrounding the optimal drug dosage, administration schedule, timing of initiation, treatment duration, and methods for monitoring adverse reactions. [8,18,19] A literature review shows no prospective study has described the optimal dosage or methods for monitoring side effects in infants and children. Hence, this study was designed to assess the safety and effectiveness of propranolol in treating IH in Libyan pediatric patients.
Study design, population, and setting
A prospective study was conducted at the Tripoli Children's Hospital hematology outpatient clinic from October 2013 to December 2016. The hospital is located at the heart of the Libyan capital. It has almost 250 beds, including 12 intensive care beds. Various outpatient clinics are available, including hematology and neonatology. The hematology clinic is a daily clinic run by a team consisting of a hematologist, a specialist, and a resident. Patients who require admission are referred to the hospital, and the hematology and general pediatric teams follow up with them.
Patients recruitment
The patients were recruited from those following the hematology clinic in Tripoli Children's Hospital. The children with IHs and their parents received an educational leaflet about the disease and available therapy options. After they understood the process, the parents gave us consent to collect the data and agreed to include the pictures and the data results in this paper.
Inclusion and exclusion criteria
Children were excluded if they had contraindications to propranolol, such as a history of allergic reactions or sensitivities, hypoglycemia, hypotension, advanced heart block (second- or third-degree), cardiac failure, severe bradycardia, asthma, chronic diseases (cardiac, kidney, or lung), or digestive system disorders that could affect propranolol intake or absorption. Furthermore, the researchers excluded children with insufficient or inconsistent follow-up visits.
Treatment initiation and follow-up process
The researchers collected data, including the child's age at first presentation, gender, wheezing history, family atopy history, lesion location, and size, as well as whether the lesion(s) was/were deep or superficial, ulcerated, infected, or bleeding before treatment. After a comprehensive discussion with parents regarding the nature of propranolol, its potential effects on the lesion, and possible side effects, the parents agreed to sign a consent form for collecting the aforementioned data and permission to publish the results without mentioning any identifying characteristics. Photographs of the lesion(s) were taken at the first visit as a reference for changes in the lesion during subsequent follow-up visits (size and color). Parents were asked to document in writing any changes in their child's health or behavior that might indicate potential propranolol-related adverse effects, providing a detailed description.
Following a clinical assessment, patients underwent electrocardiography, ultrasonography, and MRI as needed. Subsequently, propranolol was initiated for only 68 children and continued till they improved. The initial dose was 1 mg/kg/day, given in three equal doses on the first day. Blood pressure, heart rate, and blood sugar were monitored during the first 5 hours after receiving the first treatment dose as an inpatient. Then, the child was discharged and reevaluated the next day for side effects. The propranolol dose was then increased to 2 mg/kg/day on the second day, divided into equal doses every eight hours, and continued if no side effects were reported. Patients were closely monitored after starting propranolol to adjust the dosage, evaluate its effectiveness, and identify any new adverse reactions. Twelve patients had atenolol; ten of these individuals had a family history of asthma or bronchospasm, and for the other two children, one had developed bronchospasm, and one had gastroesophageal reflux features after starting propranolol. In 20 children with unusually large hemangiomas, infected lesions, or lesions near critical organs like the vocal cords and eyes, a combination of steroids and propranolol was initially administered for one month, followed by a gradual reduction in one to two months.
Statistical analysis and ethical approval
The mean, standard deviation, and frequency were computed utilizing the SPSS statistical software version 2020 along with Microsoft Excel 2021. Ethical approval for conducting the study was granted by the Tripoli Children Research Ethics Committee, and the final approval was provided by the Hospital Director.
The gender distribution comprised 62% females and 38% males. The ages of participants varied from 30 days to 60 months. Eighty-seven children were < 12 months, while 13 were between 13 and 60 months. The follow-up duration was between 5 and 19 months. (Figure 2) represents the gender of the children and the number of lesions.
.png)
Figure 2: The gender distribution and the number of lesions in the participating children.
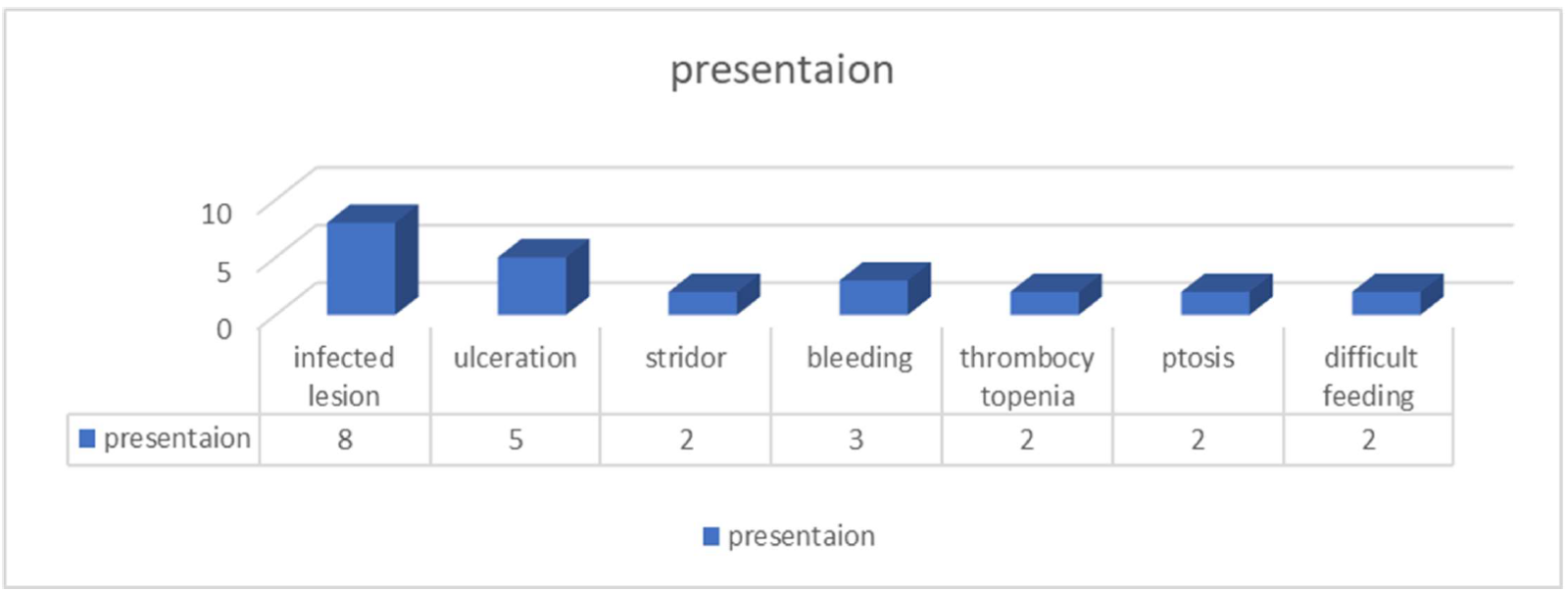
Hemangioma lesions were distributed, with 56% occurring on the face, 11% on the lips, and 6% found on the nose, specifically two at the nasal tip, 3% on the nasal sides, and 1% on the intranasal septum. Four patients exhibited ear lesions, two with pre-auricular and two with post-auricular lesions. Periorbital IH was observed in five patients, while eyelid IH occurred in four cases. Thirteen children presented with hemangiomas on their cheeks, eight on their foreheads, and one on their necks. A patient presented with an IH located on the chest. Twelve children exhibited hemangioma lesions on their extremities: four on the shoulder, one on the arm, five on the forearm, and two on the thigh. Furthermore, one lesion was identified in the inguinal region, while four were present in the perineum, comprising two skin scrotal and two perianal lesions. Nineteen children were identified with multiple hemangiomas affecting internal organs, including one in the vocal cord, liver, scrotal skin, tongue, and superficial cutaneous region. Some children presented with bleeding in three patients, feeding difficulties in two children, and infected lesions in eight children, with five of these lesions being ulcerated. One child demonstrated respiratory obstruction characterized by stridor and severe dyspnea; two children exhibited ptosis, while two others presented with thrombocytopenia. These presentations are summarized in (Figure 3).
Outcome
Collectively, 14 infants under one year of age experienced complete hemangioma disappearance, 17 children experienced near-complete disappearance, and 69 children noted a decrease in hemangioma size. The outcome is summarized in (Figure 4).
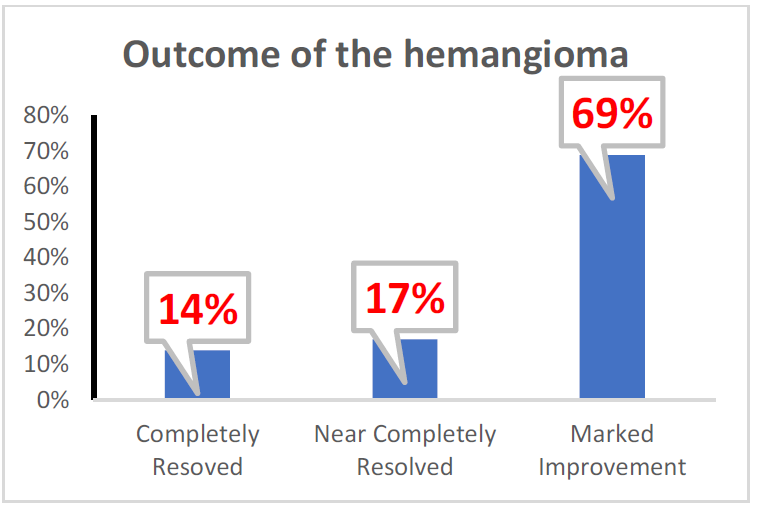
Figure 4: Summary of the hemangioma outcome.
Of the 68 children who received only propranolol, 12 children < 12 years had their hemangioma resolved completely, another 15 children had almost resolved hemangioma, and the rest (41 children) showed a significant decrease in hemangioma size without disfigurement. After six months of propranolol discontinuation, two children had a relapse of the lesion. In one child, the lesion disappeared after 12 months of restarting propranolol, and the other child's lesion required 18 months of propranolol.
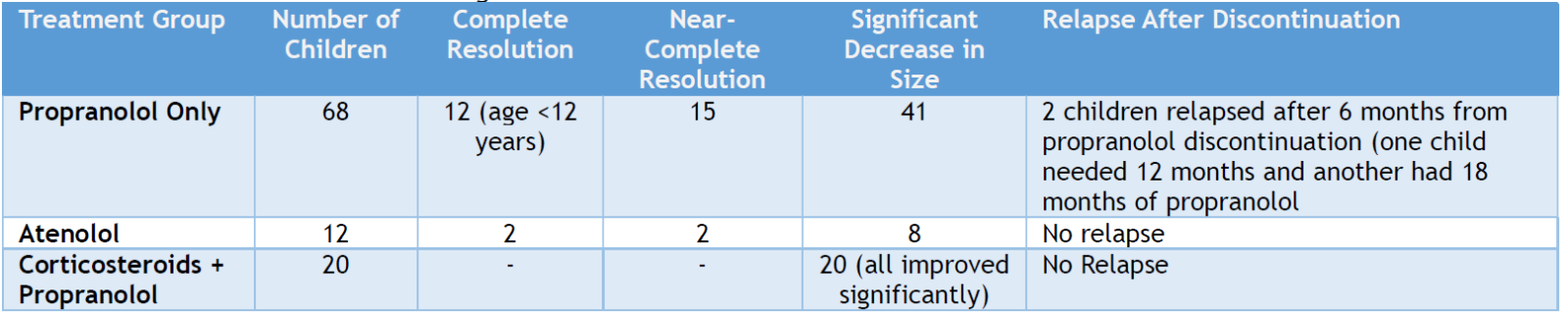
Among children who had atenolol, two had complete resolution, two had near-complete resolution, and 8 had a considerable decrease in the hemangioma lesions. No relapses or changes in the lesion sizes occurred after atenolol discontinuation. For the 20 children who received corticosteroids and propranolol, the lesions improved significantly in size. The summary of the outcome of the hemangioma lesion following the therapy, according to improvement and treatment used, is presented in (Table 1). (Figures 5-6) show examples of the degree of improvement of the hemangioma lesions.
Figures 5-6 : Shows two different children before and after beta-blocker therapy
Table 1: Treatment Outcomes of Hemangioma.
Parents or medical professionals can detect hemangioma shortly after birth, although it may develop later. Generally, infantile hemangioma is a harmless condition that resolves on its own. [2] Nevertheless, there have been instances of severe, extensive, and disfiguring hemangiomas. Prompt diagnosis and treatment are recommended and yield positive results. Recently, beta-blockers have become the preferred medical intervention for infantile hemangioma, with minimal side effects. Propranolol inhibits β1 and β2 receptors, causing peripheral vasoconstriction due to unopposed α-adrenergic receptor activity, resulting in the constriction of peripheral blood vessels. [20] When taken orally by fasting patients, propranolol is completely absorbed, reaching peak plasma levels within 1 to 2 hours. The propranolol plasma half-life is 3 to 6 hours, with the liver accounting for 90% of its elimination. Propranolol is widely used for IH treatment and effectively heals or reduces hemangioma, surpassing the treatment effect of steroid treatment. It serves as an effective alternative due to its rapid and immediate response, avoiding side effects associated with prolonged high-dose steroid therapy. [9]
Steroid is a recognized standard first-line treatment for a quick hemangioma. [9] Although the exact mechanism of propranolol's action on IH is unclear, studies suggest that vasoconstriction plays a crucial role in improving lesions, [21] and inhibits proangiogenic pathways in the proliferative phase. [22] It increases microvascular endothelial cell death while suppressing proangiogenic signals such as vascular endothelial growth factor and essential fibroblast growth factor. Propranolol treatment swiftly impairs the proliferation and promotes the regression of severe hemangioma, even after the growth phase has concluded [13] with a favorable safety profile.
Known side effects of propranolol include transient bradycardia, hypotension, hypoglycemia, hyperkalemia, diarrhea, and bronchospasm in patients with underlying reactive airways. [11,13,22] Propranolol was discontinued in 12 children (12%) due to bronchospasm; 10 children had a family history of wheezing, one child had developed wheezing, and one had gastroesophageal reflux after initiating propranolol. In these 12 patients, propranolol was substituted with atenolol. Atenolol also proved effective in treating hemangioma without significant reported bronchospasm or other complications observed with propranolol in the present study. Interestingly, the complete disappearance rate in children with propranolol or atenolol was not different (~13%).
Some physicians offer propranolol as an outpatient treatment with an initial test dose, while others require hospitalization to begin therapy. In this study, a third of the first dose of propranolol (1 mg/kg/day)was administered in the hospital; then the child was monitored for 5 hours. If there were no complications, the child was discharged and reassessed the next day for any side effects, and the dose was increased to 2 mg/Kg/day.
It was reported that propranolol can be started as an inpatient at 0.5 mg/kg/day on day one and increased to 1 mg/kg/day on day two. If no adverse effects occur, the dose is increased directly to 2 mg/kg/day orally in three divided doses every 8 hours on the third day, after which the patient is discharged. [23] Another study examined 108 patients and concluded that the average duration of treatment was longer than 12 months. The maximum dose of propranolol of 3 mg/kg/day was achieved after 3 to 5 months of treatment. Side effects occurred in 19 patients, with night anxiety and nightmares being the most common. They reported an excellent response. [24] Hence, it is generally accepted to initiate propranolol therapy with a low dose and gradually increase the dose with a slow increment.
Corticosteroid treatments are most effective during the proliferative phase, usually occurring between one and four months after the lesion's occurrence. Systemic steroids were first used to treat IH in 1960, with prednisolone doses ranging from 2-4 mg/kg/day given as a single morning dose, depending on the clinical presentation. Steroid side effects include gastrointestinal discomfort, irritability, weight gain, cushingoid appearance, hypertension, growth retardation, adrenal suppression, and immunosuppression. [25] In this study, 20 patients received steroid therapy for complications: one patient had stridor from vocal cord involvement, another experienced nasal blockage from an eye-area hemangioma, and a third had nasal obstruction from a septal hemangioma. The other 17 patients had either ulcerated or large hemangiomas near vital organs. Propranolol, in conjunction with a brief course of steroids, was highly effective in quickly reducing hemangioma size and color in the present study. The full dose of steroids lasted only for one month, then tapered and stopped after 1-2 months, while propranolol continued. The lesions treated by the combined therapy had no recurrences.
In the present study, atenolol was administered to 12 children with hemangioma, and the response was good, supporting the available reports. [26] All patients in the present study responded excellently to treatment: 73 patients experienced a reduction in hemangioma size, 12 patients achieved complete resolution, and 15 patients showed near-complete disappearance without significant reported adverse effects. Studies have associated propranolol with adverse effects, including perspiration, chilled extremities, and loose stools, in cases of hemangioma. [27]
These reactions can be attributed to oral propranolol liquid preparations, which contain varying amounts of maltitol, propylene glycol, sorbitol, ethanol, and benzyl alcohol, [17] potentially causing these side effects rather than propranolol. In the present study, most of these side effects were not reported. A minority of our participants reported thrombocytopenia. We believe that thrombocytopenia is not due to propranolol because large hemangiomas may cause thrombocytopenia by increasing platelet sequestration and destruction. [28]
In this study, we have not observed any difference in the treatment duration of the three groups (propranolol, atenolol, or the steroid plus propranolol group) in the hemangioma improvement rate. However, only two cases in the propranolol-treated group had recurrences that required a prolonged propranolol course (12-18 months), as reported in a recent report. [23] Reports have described the use of initial combination therapy with systemic corticosteroids and propranolol, resulting in rapid improvement, usually within 3 months, allowing for faster corticosteroid tapering. Comparing propranolol and steroids showed its superiority. [9] A meta-analysis study revealed that the propranolol effective rate was greater than that of steroids in treating IH (P<0.00001), with fewer propranolol complications than steroids (P<0.00001). [9]
Limitations
Although the number of patients involved is 100 children, there was a good chance that the number could be higher, providing more robust results and conclusions. A comparison of responses between the different beta-blockers used had not been conducted, which could support the superiority of propranolol's efficacy. Furthermore, a comparison between the response rate and efficacy of steroids alone and with propranolol was not conducted. The study did not have a control or comparative arm, and there are some deficiencies in the statistical analysis, such as a lack of stratified analysis. We believe these limitations must be considered carefully in future research.
Propranolol is an effective treatment for infantile hemangiomas, demonstrating low side effects and recurrence rates. However, treatment should be individualized, and further comparative studies are warranted. Nonetheless, close monitoring for bronchospasm and wheezing is essential. Atenolol is a suitable alternative due to its widespread availability, favorable safety profile, and comparable efficacy. While steroid therapy remains an option, its use should be limited due to potential adverse effects.
ACKNOWLEDGMENT
The authors acknowledge the support of the Open Libyan University for its unlimited support in conducting the study.
AUTHORS’ CONTRIBUTION
Each author has made a substantial contribution to the present work in one or more areas, including conception, study design, conduct, data collection, analysis, and interpretation. All authors have given final approval of the version to be published, agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
References
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390(10089):85-94.
- Chamli A, Aggarwal P, Jamil RT, Litaiem Nl. Hemangioma. [Updated 2023 Jun 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
- Yu Y, Flint AF, Mulliken JB, Wu JK, Bischoff J. Endothelial progenitor cells in infantile hemangioma. Blood. 2004;103(4):1373-5.
- Holland KE, Drolet BA. Infantile hemangioma. Pediatr Clin North Am. 2010;57(5):1069-83.
- Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93(6):2357-64.
- Carvalho F, Liberal M, Vale F, Rodrigues Santos N, Guedes R. Early Presentation of an Ulcerated Infantile Haemangioma in a Newborn. Cureus. 2022;14(1):e21545.
- Jacobs AH. Strawberry hemangiomas; the natural history of the untreated lesion. Calif Med. Jan 1957;86(1):8-10.
- Zheng JW, Wang XK, Qin ZP, Fan X-D, Kai Li5, Yang Y-W, et al. Chinese expert consensus on the use of oral propranolol for treatment of infantile hemangiomas (version 2022). Front Oral Maxillofac Med 2022;4:32-41.
- You Y, Li Y, Xiao Y, Zhang J. Propranolol vs. steroids in the treatment of infantile hemangiomas: A meta-analysis. Mol Clin Oncol. 2021;15(2):156.
- Bertrand J, McCuaig C, Dubois J, Hatami A, Ondrejchak S, Powell J. Propranolol versus prednisone in the treatment of infantile hemangiomas: a retrospective comparative study. Pediatric Dermatology. 2011;28(6):649-54.
- Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269–74.
- Restrepo R, Palani R, Cervantes LF, Duarte AM, Amjad I, Altman NR. Hemangiomas revisited: the useful, the unusual and the new. Part 2: endangering hemangiomas and treatment. Pediatr Radiol 2011; 41: 905–15.
- Lawley LP, Siegfried E, Todd JL. Propranolol treatment for hemangioma of infancy: risks and recommendations. Pediatr Dermatol. 2009;26:610–614.
- Zhang L, Mai HM, Zheng J, Zheng JW, Wang YA, Qin ZP, Li KL. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol. 2013;7(1):48-55.
- Roganovic J. An update on the treatment of high-risk hemangiomas in infants. Eur J Pediatr Surg. 2007;17(2):147.
- Sharma VK, Fraulin FO, Dumestre DO, Walker L, Harrop AR. Beta-blockers for the treatment of problematic hemangiomas. Can J Plast Surg. 2013;21(1):23-8.
- Tan ST, Itinteang T, Leadbitter P. Low-dose propranolol for infantile haemangioma. J Plast Reconstr Aesthet Surg. 2011;64:292-9.
- Cohen SR, Wang CI. Steroid treatment of hemangioma of the head and neck in children. Ann Otol Rhinol Laryngol. 1972;81:584–90.
- Zheng JW, Wang XK, Qin ZP, Fan XD, Li K, Yang YW, et al. Chinese experts consensus on the use of oral propranolol for treatment of infantile hemangiomas. Shanghai Kou Qiang Yi Xue. 2016;25(3):257-60.
- Khouri C, Jouve T, Blaise S, Carpentier P, Cracowski JL RM. Peripheral vasoconstriction induced by β-adrenoceptor blockers: a systematic review and a network meta-analysis. Br J Clin Pharmacol. 2016;82(2):549-60.
- Kowalska M, Dębek W, Matuszczak E. Infantile Hemangiomas: An Update on Pathogenesis and Treatment. J Clin Med. 2021 Oct 9;10(20):4631.
- Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthet Surg. 2011;64:445–451.
- Tiemann L, Hein S. Infantile Hemangioma: A Review of Current Pharmacotherapy Treatment and Practice Pearls. J Pediatr Pharmacol Ther. 2020;25(7):586-599.
- Gałązka P, Bereźnicka W, Leis K, Kaczor P, Daniluk-Matraś I, Czajkowski R, et al. Treatment of haemangiomas using propranolol in paediatric patients: a retrospective cohort study. Postepy Dermatol Alergol. 2020;37(4):603-607.
- Lomenick JP, Reifschneider KL, Lucky AW, Adams D, Azizkhan RG, Woo JG, et al. Prevalence of adrenal insufficiency following systemic glucocorticoid therapy in infants with hemangiomas. Arch Dermatol. 2009;145:262–266.
- Ballona R, Velásquez’ F, Kikushima I, Zevallos JP, Nuñez J, Apagüeño C. Atenolol use for infantile hemangiomas. Indian J Dermatol Venereol Leprol. 2021;87(2):321.
- Apfelberg DB, Maser MR, White DN, Lash H. A preliminary study of the combined effect of neodymium: YAG laser photocoagulation and direct steroid instillation in the treatment of capillary/cavernous hemangiomas of infancy. Ann Plast Surg. 1989;22(2):94-104.
- Schulz AS, Urban J, Gessler P, Behnisch W, Kohne E, Heymer B. Anaemia, thrombocytopenia and coagulopathy due to occult diffuse infantile haemangiomatosis of spleen and pancreas. Eur J Pediatr. 1999;158(5):379-83.
