Full HTML
Immunohistochemical Expression of Progesterone Receptor and B-cell Lymphoma-2 Antigen in Uterine Leiomyomas in a Southwestern Nigerian Teaching Hospital
Adebayo Ayoade Adekunle1, Olabisi Ayo-Aderibigbe1, Mumini Wemimo Rasheed2, Najeem Adedamola Idowu3, Oluwole O. Odujoko4, Donatus Sabageh1
Author Affiliation
1 Consultant, Department of Morbid Anatomy and Histopathology, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
2 Consultant, Department of Anatomic Pathology, Federal University, Dutse, Nigeria
3 Consultant, Department of Surgery, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
4 Consultant, Department of Morbid Anatomy and Forensic Medicine, Obafemi Awolowo University, Ife, Osun State, Nigeria
Abstract
Background: Uterine leiomyoma is the most common benign smooth muscle tumor of unknown aetiology. Progesterone may contribute to leiomyoma growth through the induction of B-cell lymphoma-2 (BCL-2) protein in leiomyoma cells. This study aims to determine the patterns of B-cell lymphoma-2 (BCL-2) and progesterone receptor (PR) expression in uterine leiomyomas seen at LTH, Ogbomoso, over a five-year period using immunohistochemical techniques.
Methods: This was a hospital-based retrospective study of histologically diagnosed leiomyomas in the histopathology department of a teaching hospital between January 2012 and December 2016. A total of 141 cases of uterine leiomyomas were semi-quantitatively analysed immunohistochemically for PR and BCL-2 antigens.
Results: Immunohistochemical analysis showed that out of 141 cases studied, 74 (52.5%) and 118 (83.7%) were positive for BCL-2 and PR, respectively. Among the 141 cases, 23 (16.3%) were negative for both PR and BCL-2. There was a moderate positive correlation between the immunohistochemical expression of BCL-2 and PR antigens, with a p-value < 0.001 (Pearson correlation = 0.563).
Conclusion: This study showed that the majority of women with leiomyomas expressed both progesterone receptor and B-cell lymphoma-2 antigens. Therefore, selective women with leiomyomas could benefit from progesterone receptor modulators instead of undergoing invasive procedures such as myomectomy or hysterectomy.
DOI: 10.63475/yjm.v4i1.0077
Keywords: Immunohistochemistry, BCL-2, Progesterone Receptor, Uterine Leiomyoma, Fibroids
Pages: 134-139
View: 7
Download: 7
DOI URL: https://doi.org/10.63475/yjm.v4i1.0077
Publish Date: 22-05-2025
Full Text
Leiomyomas, also known as fibroids, are benign neoplasms of the uterus that are common among women of reproductive age. Their growth varies significantly among individuals, as factors such as hormones, genetics, and pregnancy influence their development. [1,2] Typically, they grow slowly over time, with fluctuations in growth rates, particularly during the menstrual cycle, pregnancy, and menopause. [1]
More importantly, the initial event in the pathogenesis of leiomyomas remains unknown. However, it is widely believed that ovarian steroid hormones play a significant role in their development and maintenance. [2,3] Current evidence from clinical, biochemical, histological, molecular, and pharmacological studies suggests that progesterone and its receptor (PR) play important roles in the growth and development of uterine leiomyomas. [2,3] Some researchers have reported an increased concentration of PR in leiomyoma tissue compared with adjacent myometrium. [4–6] Additionally, studies have shown that progesterone suppresses apoptosis and stimulates the proliferation of leiomyoma cells in cell culture, whereas PR modulators inhibit cellular proliferation and induce apoptosis in these cells by acting on the BCL-2 gene. [7,8]
BCL-2 antigen is a product of the BCL-2 anti-apoptotic gene and was initially discovered in B-cell lymphomas. The BCL-2 gene encodes a 26-kDa protein localized in mitochondrial and perinuclear membranes. [9,10] B-cell lymphoma-2 antigen plays a crucial role in tumor growth by preventing apoptotic cell death, either by extending the lifespan of certain cells or promoting cell replication. In addition to prolonging cell survival, the BCL-2 protein can facilitate cell replication by reducing the requirement for growth factors. [10]
The interaction between progesterone and BCL-2 is an active area of research aimed at understanding the mechanisms behind fibroid development and exploring targeted treatment options. Some studies have shown that progesterone contributes to leiomyoma growth by inducing BCL-2 antigen expression in leiomyoma cells. [5,11] In Nigeria, there is a paucity of information on the expression of BCL-2 protein and PR in uterine leiomyomas. To address this gap, this study will investigate the expression of BCL-2 and progesterone receptors in uterine leiomyomas at Ladoke Akintola University Teaching Hospital (LTH), Ogbomoso. The aim is to determine the patterns of PR and BCL-2 expression in leiomyomas over five years using immunohistochemical techniques.
Study design, population, and setting
This study was a five-year retrospective cross-sectional analysis of all histologically diagnosed cases of uterine leiomyomas at the Department of Morbid Anatomy, Ladoke Akintola University Teaching Hospital (LTH), Ogbomoso, Oyo State, Nigeria, between January 2012 and December 2016. LTH is a tertiary health institution located in the Southwest geopolitical zone of Nigeria and serves as a referral center for Oyo, Kwara, and Osun States. The Department of Morbid Anatomy provides routine histopathology services and has the expertise and resources for diagnostic tissue processing. Immunohistochemical analyses were carried out at the Department of Pathology, University of Ilorin Teaching Hospital (UITH), Ilorin, Kwara State, where the required facilities were available.
Inclusion and exclusion criteria
All cases of histologically confirmed uterine leiomyomas diagnosed during the study period were considered. Cases with poor tissue preservation, incomplete clinical data, or inadequate tissue samples for immunohistochemistry were excluded from the study.
Sample size calculation
A sample size calculation was carried out using the finite population correction formula to determine the minimum number of uterine leiomyoma cases required for immunohistochemical analysis.
Yamane formula was used: n=N/1+N.e2
Where:
n = sample size
N = population size (total number of histologically confirmed uterine leiomyomas during the study period) = 215
e = precision = 0.05 (5%)
The calculated minimum sample size was approximately 139. However, to improve the reliability of results and account for possible tissue loss or inadequate staining, the sample size was adjusted to 141 cases. Only the most representative formalin-fixed paraffin-embedded (FFPE) tissue blocks were selected for immunohistochemical studies.
Sampling was guided by the inclusion and exclusion criteria to ensure quality and consistency in analysis.
Specimen processing and immunohistochemistry
Archived Hematoxylin and Eosin-stained slides were reviewed, and representative FFPE tissue blocks were retrieved. Immunohistochemical staining was performed using the avidin-biotin-peroxidase complex technique. Monoclonal antibodies used included PR mouse monoclonal antibodies and BCL-2 mouse monoclonal antibodies (Elabscience, USA). Semi-quantitative evaluation of immunostaining was performed using the Allred scoring system. [12,13]
Statistical analysis
Data were analyzed using IBM SPSS version 23.0 (IBM Corp, Armonk, NY, USA). Descriptive statistics were used to summarise patient demographics and immunohistochemical results. The Pearson correlation coefficient was used to assess the relationship between PR and BCL-2 expression. A p-value of less than 0.05 was considered statistically significant.
Ethical considerations
Ethical approval for this study was obtained from the Ethical Review Committee of LTH, Ogbomoso (Approval Reference Number: LTH/OGB/EC/2017/155). This study used archived anonymized tissue samples; therefore, informed consent was not applicable. All patient data were handled with strict confidentiality, and all datasets were securely stored on a password-protected computer system. The study adhered to the ethical standards outlined in the Declaration of Helsinki.
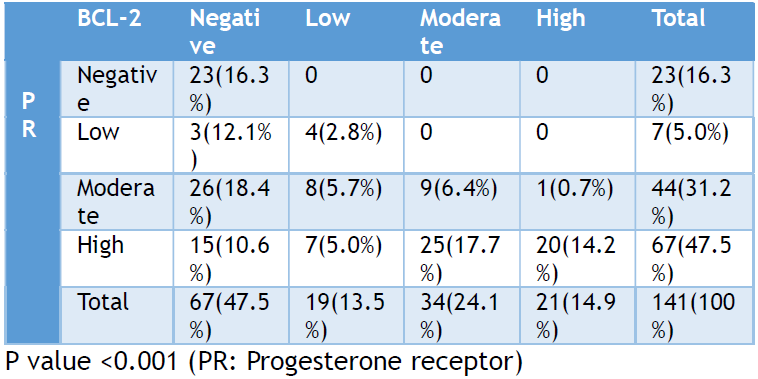
The study included 141 cases of uterine leiomyomas, with patients ranging in age from 26 to 85 years. The mean age at presentation was 40.44 years (±9.96 SD). Among the cases studied, 67 (47.5%) were negative for BCL-2 immunoexpression, while 19 (13.5%), 34 (24.1%), and 21 (14.9%) cases showed low, moderate, and high immunoreactivity, respectively (Figure 1). For PR expression, 23 (16.3%) cases were negative, while 7 (5.0%), 44 (31.2%), and 67 (47.5%) cases showed low, moderate, and high expression, respectively (Table 1).
.png)
Figure 1. Graph showing the relationship between age, progesterone receptor, and BCL-2.
.png)
Figure 2. Photomicrograph showing immunopositivity for progesterone receptor in uterine leiomyoma with brownish nuclear immunostain. (IHCx40). Figure 3. Photomicrograph showing immunopositivity for B-cell lymphoma-2 antigen in uterine leiomyoma with brownish cytoplasmic immunostain. (IHCx40).
(Table 1) summarizes the immunoreactivity of PR and BCL-2 in uterine leiomyomas. A total of 23 (16.3%) cases were negative for both PR and BCL-2, while 74 (52.5%) cases showed positive expression for both markers. A moderate positive correlation was observed between the immunohistochemical expression of PR and BCL-2, with a Pearson correlation coefficient of 0.563 and a statistically significant p-value of <0.001.
Table 1. Immunoreactivity of progesterone receptor and b-cell lymphoma-2 antigens expressed in uterine leiomyomas studied between 2012 and 2016
In leiomyomas, there is a complex relationship between BCL-2 and progesterone. Progesterone, a reproductive hormone, influences fibroid growth by promoting cellular proliferation and regulating gene expression. BCL-2, an anti-apoptotic protein involved in cell survival, may be influenced by progesterone signaling pathways within tumor cells, potentially contributing to their growth and persistence. [3,5]
Among reproductive-age women, the relationship between BCL-2 and progesterone in leiomyomas has been documented in several studies. [5,14–17] During the menstrual cycle, progesterone levels fluctuate, affecting the growth and biology of leiomyoma cells. These hormonal changes may influence BCL-2 expression, contributing to the growth and maintenance of leiomyomas. [16,17]
This study found that progesterone receptors (PR) and BCL-2 antigens were expressed in 83.7% and 52.5% of uterine leiomyomas, respectively. Additionally, 74 patients (52.5%) expressed both PR and BCL-2, and a moderate correlation was observed between the immunohistochemical expression of BCL-2 and PR antigens in uterine leiomyomas, with a p-value of less than 0.001. This suggests that PR and BCL-2 expression play a significant role in leiomyoma development. Various studies have shown that PR promotes the growth of leiomyomas by upregulating BCL-2 expression in leiomyoma cells. [5,7,18] Yin et al demonstrated that the human BCL-2 promoter region binds to progesterone and regulates BCL-2 expression in leiomyoma cells. [7] Similarly, Matsuo et al observed that progesterone may contribute to leiomyoma growth by inducing BCL-2 expression. [5] Furthermore, studies have shown that progesterone receptor modulators mediate growth inhibition and apoptosis in leiomyoma cells by reducing BCL-2 expression. [6,19] Islam et al also highlighted that selective progesterone receptor modulators (SPRMs), such as mifepristone and ulipristal acetate can reduce fibroid volume and symptoms by modulating BCL-2 expression and induce apoptosis in leiomyoma cells. [19]
In this study, 23 (16.3%) of the leiomyomas reviewed did not express either PR or BCL-2 antigens. This finding suggests that other mechanisms are involved in leiomyoma growth and development. Research has shown that progesterone and its receptor play roles in various pathways of tumorigenesis. [3,20] One such pathway is oestrogen-progesterone signaling. [3] Ishikawa and colleagues found that oestradiol induces PR expression in leiomyoma cells. [18] Besides estrogen-progesterone interaction, Maruo and his group in Japan found that progesterone downregulates insulin-like growth factor-I (IGF-1) expression in human leiomyoma cells. [21] Their findings suggest that growth factor signaling may interact with progesterone signaling in tumorigenesis. Other studies have also concluded that receptors for various growth factors, such as insulin, insulin-like growth factor, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-β), are expressed in leiomyomas and may contribute to tumor growth. [15,22,23] Additionally, in leiomyoma cells, progesterone, through its receptor, can activate the PI3K/AKT pathway, and an AKT inhibitor has been shown to promote apoptosis in leiomyoma cells despite the presence of progestins. [6] The findings of this study and the literature suggest that progesterone signaling and apoptosis via BCL-2 expression are part of the complex pathways involved in leiomyoma development. [3,15]
B-cell lymphoma-2 (BCL-2) is unique in being localized to the inner mitochondrial membrane, where it functions as an oncogene by blocking programmed cell death. [9,10] Various agents that interfere with BCL-2 or other anti-apoptotic BCL-2 family proteins act as chemo-sensitizers, making it easier for conventional anti-tumor drugs to induce cell death. [9, 24,25] In view of this, Vogler et al emphasized that the BCL-2 family proteins are central regulators of apoptosis, and their dysregulation can lead to tumorigenesis, making them potential targets for therapeutic intervention in leiomyomas. [9] Castro et al also demonstrated that high concentrations of genistein induce autophagic cell death in human uterine leiomyoma cells, suggesting that targeting autophagy pathways could be a novel therapeutic approach. [24] Ura et al identified specific phosphoproteins involved in the inhibition of apoptosis and promotion of cell survival in leiomyomas, highlighting potential molecular targets for therapy. [26]
Furthermore, drugs such as selective progesterone receptor modulators (SPRMs), vitamin D, and epigallocatechin gallate act by modulating PR or repressing BCL-2 gene expression in leiomyomas. [19,26–29] These drugs could be beneficial for leiomyomas that stain positively for both PR and BCL-2, offering an alternative to surgical interventions such as hysterectomy and myomectomy, which may be associated with life-threatening complications. [26] Medical management of leiomyoma was also discussed by Laughlin-Tommaso and Stewart, who advocated for individualized medicine approaches in the management of uterine leiomyomas, emphasizing the need for personalized therapies based on the molecular profile of the tumors to improve patient outcomes. [30]
It is, therefore, essential to identify patients who could benefit from such medical therapies using immunohistochemistry for PR and BCL-2 on biopsy specimens. This approach could reduce the risk of complications associated with surgical treatment. Consequently, immunohistochemistry should be considered an ancillary investigation in the diagnosis and management of uterine leiomyomas. Patients with positive immunostaining for PR and BCL-2, especially those with recurrent nodules after myomectomy or those who prefer medical treatment, could benefit from progesterone receptor modulators.
Limitations and Strengths of the Study
A limitation of this study is that it was conducted in a single center, which may limit the generalizability of the findings. Its retrospective design relied on archival tissues and incomplete clinical data, which may affect result accuracy. The absence of normal myometrial controls in all cases, lack of tumor stratification, and reliance on manual immunohistochemistry scoring could introduce bias and reduce precision. Additionally, other molecular markers that could further elucidate leiomyoma pathogenesis were not evaluated. Also, the study period (2012–2016) may be considered outdated; it still offers significant insight into the expression of BCL-2 and PR in leiomyomas. Future research using advanced diagnostic methods may further validate and expand upon these findings.
Despite these limitations, the study's strength lies in its provision of immunohistochemical data on PR and BCL-2 expression in leiomyomas, contributing valuable insights into the molecular mechanisms underlying leiomyoma development. This study also highlights the potential role of immunohistochemistry in guiding non-surgical treatment options for uterine leiomyomas. It also serves as a valuable baseline for future research and supports the need for larger, multi-center studies incorporating advanced molecular methods.
This study demonstrated that the majority of women with leiomyomas expressed both progesterone receptor and BCL-2 antigens. Therefore, selective women with leiomyomas could benefit from progesterone receptor modulators instead of undergoing invasive procedures such as myomectomy or hysterectomy.
Immunohistochemical staining for PR and BCL-2 should be considered in clinical practice to guide treatment decisions for women with uterine leiomyomas. Women diagnosed with leiomyomas should receive comprehensive counseling on both medical and surgical treatment options, considering their immunohistochemical profile. Patients with positive PR and BCL-2 immunostaining should be considered for medical therapy using progesterone receptor modulators as an alternative to surgical interventions. Pathology laboratories should incorporate immunohistochemistry into their routine diagnostic protocols for uterine leiomyomas to enhance treatment stratification.
Future studies should involve multiple centers to validate findings across different populations and healthcare settings. The establishment of a national leiomyoma (fibroid) registry is recommended to collect data on various factors contributing to leiomyoma growth and development, facilitating future research. This is to investigate various molecular pathways involved in leiomyoma pathogenesis to identify new therapeutic targets. Also, additional biomarkers beyond PR and BCL-2 should be studied to provide a more comprehensive understanding of leiomyoma development and potential targeted therapies, which should be affordable and effective medical therapies for leiomyomas, particularly in resource-limited settings.
ACKNOWLEDGEMENT
This study is an extract from a dissertation submitted to the National Postgraduate Medical College of Nigeria in partial fulfillment of the requirements for the award of a Fellowship in Anatomical Pathology.
AUTHORS’ CONTRIBUTION
Each author has made a substantial contribution to the present work in one or more areas including conception, study design, conduct, data collection, analysis, and interpretation. All authors have given final approval of the version to be published, agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
SOURCE OF FUNDING
None
CONFLICT OF INTEREST
None
References
- Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):571-88.
- Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344-55.
- Kim JJ, Sefton EC. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol. 2012;358(2):223-31.
- Awowole IO, Makinde ON, Badejoko OO, Omoniyi-Esan GO, Tijani AM, Ajenifuja KO, et al. Clinical correlates of leiomyoma estrogen and progesterone receptors among Nigerian women. Int J Gynaecol Obstet. 2016;135(3):314-8.
- Matsuo H, Maruo T, Samoto T. Increased expression of BCL-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82(1):293-9.
- Hoekstra AV, Sefton EC, Berry E, Lu Z, Hardt J, Marsh E, et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab. 2009;94(5):1768-74.
- Yin P, Lin Z, Cheng YH, Marsh EE, Utsunomiya H, Ishikawa H, et al. Progesterone receptor regulates BCL-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J Clin Endocrinol Metab. 2007;92(11):4459-66.
- Huang SC, Tang MJ, Hsu KF, Cheng YM, Chou CY. Fas and its ligand, caspases, and BCL-2 expression in gonadotropin-releasing hormone agonist-treated uterine leiomyoma. J Clin Endocrinol Metab. 2002;87(10):4580-6.
- Vogler M, Braun Y, Smith VM, Westhoff MA, Pereira RS, Pieper NM, et al. The BCL2 family: from apoptosis mechanisms to new advances in targeted therapy. Signal Transduct Target Ther. 2025;10(1):91.
- Desouky MK, Anwar RI, Algaidi SA. Immunohistochemical expression of BCL-2 and microvessel density in uterine fibroids in Saudi patients. West Indian Med J. 2015;2(2):121-6.
- Liao S, Mi HN, Chai LY, Wang HN. Effects of progesterone receptor on proliferation of uterine leiomyoma cells. J Biol Regul Homeost Agents. 2019;33(6):1685-93.
- Kabirat J, Gupta J, Khaitan T, Bhattacharya PT. Principles and techniques of immunohistochemistry: A review. Int J Biol Med Res. 2015;6(4):5204-10.
- Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breastcancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155-68.
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130-62.
- Machado-López A, Simón C, Mas A. Molecular and cellular insights into the development of uterine fibroids. Int J Mol Sci. 2021;22(16):8483.
- Yun BS, Seong SJ, Cha DH, Kim JY, Kim ML, Shim JY, et al. Changes in proliferating and apoptotic markers of leiomyoma following treatment with a selective progesterone receptor modulator or gonadotropin-releasing hormone agonist. Eur J Obstet Gynecol Reprod Biol. 2015;191:62-7.
- Wu X, Blanck A, Olovsson M, Möller B, Favini R, Lindblom B. Apoptosis, cellular proliferation and expression of p53 in human uterine leiomyomas and myometrium during the menstrual cycle and after menopause. Acta Obstet Gynecol Scand. 2000;79(5):397-404.
- Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential formaintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433-42.
- Islam MS, Afrin S, Jones SI, Segars J. Selective Progesterone Receptor Modulators-Mechanisms and Therapeutic Utility. Endocr Rev. 2020;41(5):bnaa012.
- Borahay MA, Al-Hendy A, Kilic GS, Boehning D. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Mol Med. 2015;21(1):242-56.
- Maruo T, Matsuo H, Samoto T, Shimomura Y, Kurachi O, Gao Z, et al. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids. 2000;65(10-11):585-92.
- Voronin D, Sotnikova N, Rukavishnikov K, Malyshkina A, Nagornii S, Antsiferov Y. Differential regulatory effect of progesterone on the proliferation and apoptosis of uterine leiomyoma tissue explants and primary leiomyoma cell cultures. JBRA Assist Reprod. 2021;25(4):540-8.
- Gao Z, Matsuo H, Wang Y, Nakago S, Maruo T. Up-regulation by IGF-I of proliferating cell nuclear antigen and BCL-2 protein expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2001;86(11):5593-9.
- Castro L, Gao X, Moore AB, Yu L, Di X, Kissling GE, et al. A high concentration of genistein induces cell death in human uterine leiomyoma cells by autophagy. Expert Opin Environ Biol. 2016;5(Suppl 1):S1-003.
- Farris M, Bastianelli C, Rosato E, Brosens I, Benagiano G. Uterine fibroids: an update on current and emerging medical treatment options. Ther Clin Risk Manag. 2019;15:157-78.
- Ura B, Monasta L, Arrigoni G, Battisti I, Licastro D, Di Lorenzo G, et al. Phosphoproteins involved in the inhibition of apoptosis and in cell survival in the leiomyoma. J Clin Med. 2019;8(5):691.
- Catherino WH, Malik M, Driggers P, Chappel S, Segars J, Davis J. Novel, orally active selective progesterone receptor modulator CP8947 inhibits leiomyoma cell proliferation without adversely affecting endometrium or myometrium. J Steroid Biochem Mol Biol. 2010;122(4):279-86.
- Zhu Y, Xie SW, Zhang JF, Zhang TT, Zhou JY, Cao Y, et al. Involvement of BCL-2, Src, andERα in gossypol-mediated growth inhibition and apoptosis in human uterine leiomyoma and myometrial cells. Acta Pharmacol Sin. 2010;31(12):1593-603.
- Luo X, Yin P, Coon VJS, Cheng YH, Wiehle RD, Bulun SE. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril. 2010;93(8):2668-73.
- Laughlin-Tommaso SK, Stewart EA. Moving toward individualized medicine for uterine leiomyomas. Obstet Gynecol. 2018;132(4):961-71.
