Full HTML
Role of octreotide in the management of chylous pleural effusion in children: Experience with two cases
Sandip Kumar Rahul1, Rupesh Keshri1, Digamber Chaubey2
Author Affiliation
1Pediatric Surgeon, Department of Paediatric Surgery, All India Institute of Medical Sciences, Deoghar, Jharkhand,
2Pediatric Surgeon, Department of Paediatric Surgery, All India Institute of Medical Sciences, Patna, Bihar, India
Abstract
We would like to report the successful use of octreotide in the treatment of chylothorax secondary to non-cardiac surgery in two children. We would also like to emphasize that conservative treatment with chest tubes, parenteral nutrition, and octreotide is simple and is the initial treatment in most cases, whereas surgical intervention is only required secondarily.
DOI: 10.32677/yjm.v3i1.4432
Pages: 66-67
View: 7
Download: 7
DOI URL: https://doi.org/10.32677/yjm.v3i1.4432
Publish Date: 11-05-2024
Full Text
We would like to report the successful use of octreotide in the treatment of chylothorax secondary to non-cardiac surgery in two children. We would also like to emphasize that conservative treatment with chest tubes, parenteral nutrition, and octreotide is simple and is the initial treatment in most cases, whereas surgical intervention is only required secondarily.
The first case involved a 1-year-old girl with type C esophageal atresia who presented with symptoms of dysphagia and weight loss. A contrast study and endoscopy revealed a stricture at the anastomotic site, requiring right posterolateral thoracotomy and stricturoplasty to be performed because an attempt at endoscopic dilatation failed. Over the next 24 h, the chest drain output turned milky, and the biochemical analyses revealed chylothorax. The patient was kept fasting, and subcutaneous octreotide was started at a dose of 10 μg/kg body weight 3 times a day. The drain output gradually decreased, and the chest X-ray cleared. Octreotide was gradually tapered after the 7th day and stopped on the 11th day. The chest tube was removed on the 13th post-operative day, and the patient was discharged on a diet rich in medium-chain triglycerides.
The second case was a 1.5-year-old female who underwent surgery for a residual cystic hygroma involving the left neck, chest, and shoulder, following serial intralesional bleomycin therapy. This mass compressed the left internal jugular vein and extended to the main pulmonary artery, surrounding the aortic arch, and the left innominate vein with outward displacement of the trachea and esophagus. She developed respiratory distress and tachypnea on the 3rd post-operative day and on the 6th day after surgery, her chest tube showed a milky fluid which on biochemical analysis confirmed the diagnosis of left chylothorax. Subcutaneous administration of octreotide at 10 μg/kg body weight was initiated, and she was initially kept fasting and later switched to medium-chain triglyceride feed through a nasogastric tube. The chest tube drainage decreased over the next 10 days. Octreotide was tapered gradually, and the chest tube was removed on the 19th post-operative day. Figure 1 summarizes the daily drain output improvement achieved in both cases.
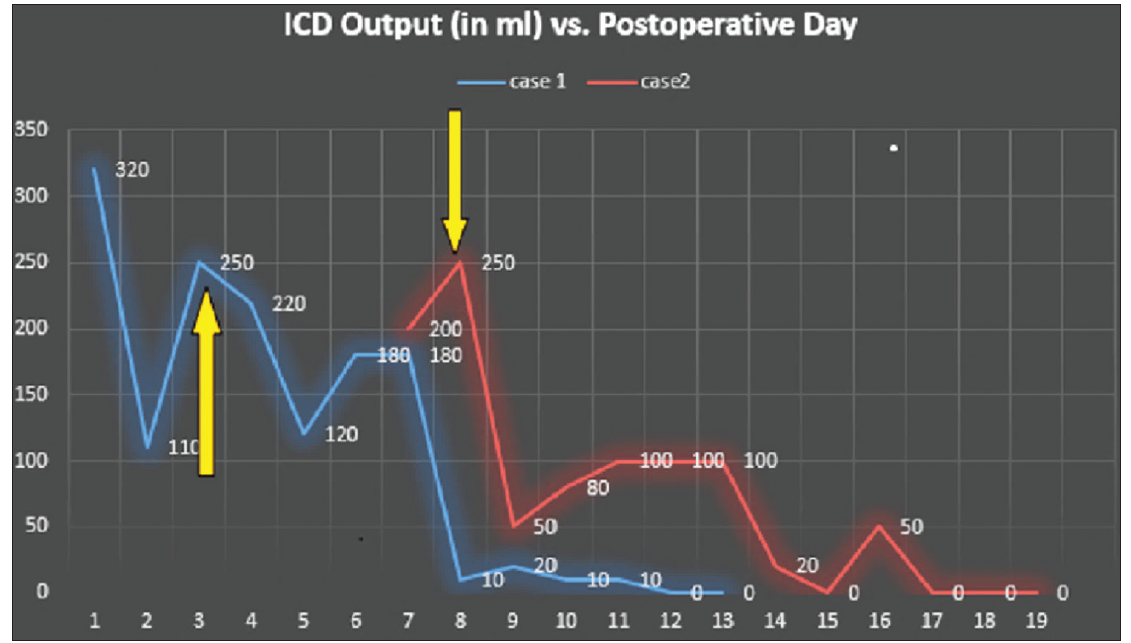
Figure 1: Line diagram showing the gradual decrease in drain output in chylothorax with the use of octreotide. Orange arrows indicate the
date of starting octreotide therapy in both cases
When the pleural fluid analysis shows a triglyceride level >1.1 mmol/L and a total cell count >1000 cells/ml with more than 80% lymphocytes, the diagnosis of chylothorax is likely. Although it most commonly occurs after cardiac surgery, chylothorax can develop after any thoracic surgery and, if left untreated, can lead to hypoalbuminemia, electrolyte imbalances, coagulation disorders, and immunodeficiency [1]. Management of chylothorax essentially involves the treatment of respiratory compromise, nutritional support, and closure of the chylous leak [2]. In cases of chylothorax diagnosed antenatally, thoracentesis or pleuroamniotic shunts to prevent pulmonary hypoplasia are needed. In the postnatal period, the management of the pleural effusion can be either conservative or surgical. The conservative approach includes management of underlying disease, repeated thoracentesis, continuous drainage, dietary modifications (medium-chain triglyceride diet or total parental nutrition) [3,4], use of positive end-expiratory pressure during mechanical ventilation [5], and chemical or mechanical pleurodesis. The surgical approach is indicated only in refractory chylothoraces and includes thoracoscopic pleurodesis, pleuroperitoneal shunts, surgical abrasion, ligation of the thoracic duct, and creation of a thoracic duct for azygous vein anastomosis [6,7].
Octreotide, a long-acting somatostatin analog, has been shown to decrease gastrointestinal secretions. It is a peptide hormone produced by paracrine cells located throughout the gastrointestinal tract, lungs, and pancreas and also found in various locations throughout the nervous system. The action of octreotide in the gastrointestinal tract is to reduce splanchnic venous pressure and inhibit the production of serotonin, gastrin, vasoactive intestinal peptide, secretin, motilin, and pancreatic polypeptide. Gallbladder contractility and bile secretion are also reduced. The drug’s exact mechanism of action is not completely understood, but it is thought to be related to reductions in hepatic venous pressure, intestinal lipid absorption, thoracic triglyceride concentrations, and splanchnic blood flow. It has been used in conditions such as gastrointestinal and pancreatic fistulas, gastrointestinal endocrine malignancies, gastrointestinal bleeding, acromegaly, and chronic diarrhea. Its effectiveness in chylothorax has been variously reported by different investigators. In 2001, Cheung et al. first reported the successful use of subcutaneous octreotide in chylothorax patients [8]. Since then, several other successful results have been reported [9-11]. Stefanidis et al., however, have failed to observe the benefits of adding octreotide to the conservative regimen [12]. In a narrative review where Habas et al. described the pathophysiology of chylous pleural effusion, the potential benefits of different modalities, including octreotide, in its management have also been highlighted [13].
These two cases were seen after non-cardiac thoracic surgeries and showed the effectiveness of subcutaneous octreotide in treating chylothorax in these patients without any significant adverse effects. It is easy to administer by the subcutaneous route and avoids unnecessary and difficult surgeries, thereby lessening the morbidity and cost of re-exploration. Although the chylothorax responded dramatically to octreotide in these two cases, such an adequate response could also be attributed to spontaneous recovery or other unidentified factors. Therefore, further research, particularly randomized controlled trials, is needed to conclusively substantiate its effectiveness.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the parents for the publication of this report.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the completion of this work. The final manuscript was read and approved by all authors.
References
- Sahin Y, Aydin D. Congenital chylothorax treated with octreotide. Indian J Pediatr. 2005;72(10):885-8.
- Ryckman F, Rosenkrantz J. Thoracic surgical problems in infancy and childhood. Surg Clin North Am 1985; 65:1423–54.
- Dubin PJ, Kind IN, Gallagher PG. Congenital chylothorax. Curr Opin Pediatr 2000; 12:505–9.
- Alvarez JR, Kalache KD, Grauel EL. Management of spontaneous congenital chylothorax: oral medium chain triglycerides versus total parenteral nutrition. Am J of Perinatol 1999; 16:415–20
- Kugelman A, Gonen R, Bader D. Potential role of high-frequency ventilation in the treatment of severe congenital pleural effusion. Pediatr Pulmonol 2000; 29:404–8.
- Selle JG, Snyder III WH, Schreiber JT. Chylothorax: indications for surgery. Ann Surg 1973; 177:245–9.
- Engum SA, Rescoria FJ, West KW, et al. The use of pleuroperitoneal shunts in the management of persistent chylothorax in infants. J Pediat Surg 1999; 34:286–90.
- Cheung Y, Leung M, Yip M. Octreotide for treatment of postoperative chylothorax. J Pediatr 2001; 139:157–9.
- Pratap U, Slavik Z, Ofoe V, et al. Octreotide to treat post-operative chylothorax after cardiac operations in children. Ann Thorac Surg 2001; 72:1740–42.
- Rosti L, Bini RM, Chessa M, et al. The effectiveness of octreotide in the treatment of post-operative chylothorax. Eur J Pediatr 2002; 161:149-50.
- Al-Zubairy SA, Al-Jazairi AS. Octreotide as a therapeutic option for management of chylothorax. Ann Pharmacother 2003; 37:679-82.
- Stefanidis C, el Nakadi I, Huynh CH, et al Benign thoracic schwannoma and postoperative chylothorax: case report and review of the of the literature. Acta Chir Belg 1994;94,105-109.
- Habas E, Farfar K, Errayes A, et al. Updates on the pathophysiology and therapies of chylous pleural effusion: A narrative review. Yemen J Med. 2023;2(3):145-150.
